Recently updated on September 19th, 2022 at 11:58 am
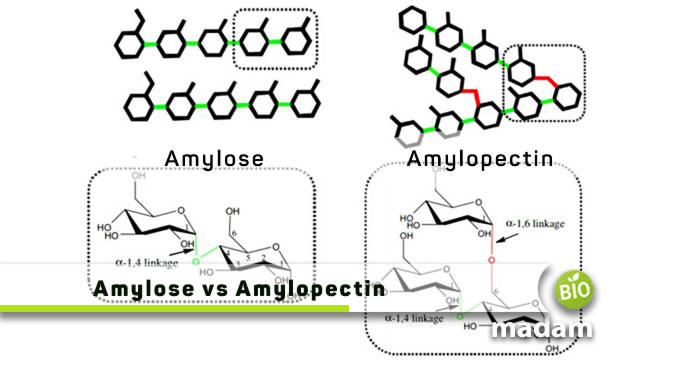
Amylose and amylopectin are both kinds of starch. Starch is a polysaccharide formed by ten or more monosaccharides. It is the primary carbohydrate, besides proteins and fats, taken by humans. They are the major energy storage in vascular as well as non-vascular plants. The main difference between amylose and amylopectin is their structure, affecting their function. You can see long chains of monosaccharides under a student microscope.
The monosaccharides are arranged linearly in amylose and arranged in a branched pattern in amylopectin. Let’s tell you about other differences between the two.
Comparison Table
| Characteristics | Amylose | Amylopectin |
| Starch Composition | 20% | 80% |
| Linkage | α 1-4 glycosidic linkages | α 1-4 & α 1-6 glycosidic linkages |
| Units | 20,000 | 2,000,000` |
| Enzyme Activity | Yes | No |
| Solubility | Insoluble | Comparatively soluble |
| Iodine Reaction | Blue | Reddish brown |
What is Amylose?
Amylose results from the linear binding of glucose units to form polysaccharides. It is made of 15 to 20% starch. Amylose contains 200 to 1000 α-D-(+)-glucose chains, together held by C1 – C4 glycosidic linkage. The OH group is removed along with an H atom from the fourth carbon of another molecule. It results in a condensation reaction involving the H and OH groups forming water.
While commonly amylose has up to 3000 repeated units, they may be in thousands. It is a water-soluble compound. α- amylase can be used to hydrolyze it into glucose units. Amylose is well-known for its binding with iodine. Amylose is chosen as a ligand as it gives a specific blue color on reaction.
Uses of Amylose
It is an essential part of energy storage in plants. It takes less space than amylopectin and provides instant energy. Amylose is often used as a binding agent on fried foods to absorb less oil and give a crispy texture. It is also used in filmmaking, paper making, textiles, and plastic products.
Examples of Amylose
Some common examples of amylose are rice, potatoes, quinoa, oats, lentils, corn, etc.
What is Amylopectin?
As opposed to amylose, amylopectin is a branched-chain polymer composed of monosaccharides. Monosaccharides are glucose molecules. The monosaccharides bind together through α 1-4 or α 1-6 glycosidic linkages. The α 1-6 glycosidic linkages give the branched structure to amylopectin. The glucose molecules bind to each other on the fourth and sixth carbon atoms. The chains consist of up to 200,000 units, and each branch contains around 30 units. Amylopectin molecules are larger than amylose.
Amylopectin comprises around 80% of the starch. It is less soluble in water and difficult to break down. Unlike amylose, α amylase and β amylase enzymes cannot hydrolyze α 1-6 glycosidic linkages. Amylopectin gives a reddish-brown color when it interacts with an iodine solution.
Uses of Amylopectin
Amylopectin can be easily digested and acts as an instant energy source. It is widely used in manufacturing lubricants and adhesives because of its starch retrogradation and binding properties.
Examples of Amylopectin
Russet potatoes and long-grain rice contain amylopectin.
Similarities Between Amylose and Amylopectin
- Amylose and amylopectin are monosaccharide polymers.
- They are made of D-glucose with α 1-6 glycosidic linkages.
- They are a part of starch.
- They both act as energy sources.
Differences Between Amylose and Amylopectin
Definition
Amylose
Amylose is a linear chain polymer of monosaccharides.
Amylopectin
Amylopectin is composed of branched chains of monosaccharides linked together.
Composition
Amylose
Amylose makes up 20% of starch.
Amylopectin
Amylopectin accounts for 80% of starch.
Linkages
Amylose
Amylose is made of α 1-4 glycosidic linkages.
Amylopectin
Amylopectin constitutes α 1-4 glycosidic linkages as well as α 1-6 glycosidic linkages, giving it the branched structure.
Number of Units
Amylose
Amylose may be composed of 3000 repeated units or more.
Amylopectin
Amylopectin may contain up to 200,000 units of glucose bound together to form a branched chain.
Solubility
Amylose
Amylose does not dissolve in room temperature water. It may dissolve in hot water, forming a gel-like substance on cooling.
Amylopectin
Amylopectin dissolves better in water.
Enzyme Activity
Amylose
You can hydrolyze amylose into glucose units through α amylase and β amylase enzymes.
Amylopectin
Amylopectin cannot be hydrolyzed with α amylase and β amylase enzymes completely.
Reaction with Iodine
Amylose
Amylose gives a blue color on interaction with iodine.
Amylopectin
Amylopectin turns reddish brown when iodine is added.
The Bottom Line
Amylose and amylopectin are starch molecules that act as an energy reserve. Amylose has a linear structure, whereas amylopectin comprises a branched chain. They are made of hundreds of monosaccharide units. Amylopectin dissolves easily in water. Alternatively, amylose dissolves only in hot water. Amylose gives a blue color to iodine, whereas amylopectin turns the solution reddish brown. High carbohydrates in your daily calorie count can cause diabetes. Similarly, low carbs can lead to hypoglycemia.
FAQs
What is the difference between amylose and amylopectin structure?
Amylose comprises a linear, helical chain of up to 20,000 glucose units. On the other hand, amylopectin has a branched-chain structure with two million glucose monomers.
What is the difference in the structure of amylose and cellulose?
The major difference in the structure of amylose and cellulose is the kind of bond. Glucose units in amylose are bound together with α 1-4 glycosidic linkages, whereas cellulose has β-1–4 glycosidic bonds.
What are amylose and amylopectin digestion?
While it may look like amylose should be easy to break down, amylopectin is easier to digest because of its structure.
Read more:
- https://www.biomadam.com/compounds-vs-mixture
- https://www.biomadam.com/molecules-vs-compound

Hi, my name is Eva. I am currently practicing as a clinical social worker, that being my childhood desire. As a licensed therapist holding MPhil in Clinical Psychology, I am now on biomadam to provide the natives with the best family advice! Do you have any questions? See you in the comment section.