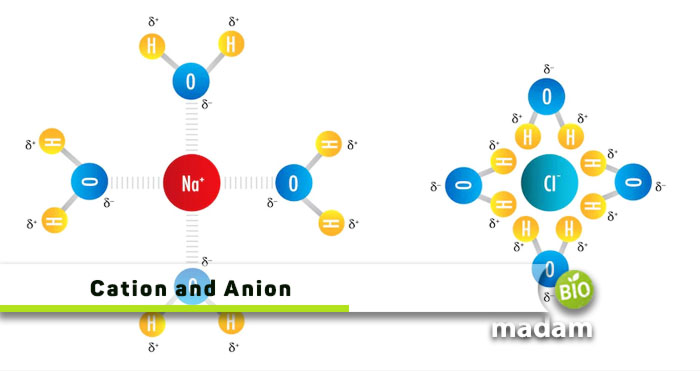
Conduction and insulation are important elements of research, chemistry, and physics experiments in school labs. They allow students and researchers to study the electric current and the movement of cations and anions. The main difference between cations and anions is the difference in charge of both ions. Cations have a positive charge while anions have a negative charge. Thus, they react with each other to produce neutral molecules and compounds.
Keep reading to learn the differences between cations and anions.
Comparison Table
| Characteristics | Cations | Anions |
| Definition | Positively charged ions | Negatively charged ions |
| Literal Meaning | Going down | Going up |
| No of Electrons | Lower | Higher |
| Attraction Force | Higher | Lower |
| Type of Element | Alkali and alkaline earth metals | Non-metals |
| Size | Small | Large |
What are Ions?
The word ion came from a word in the Greek language, which means “going.” Michael Faraday first used it in 1834. He was an English physicist and chemist. An ion is from a charged family, which includes all negative and positive charge elements. It can be defined as an atom or molecule containing an electric charge. These ions are attracted toward the cathode or anode in an electrochemical cell.
In an atom, the number of electrons and protons charges is equal, which means that they contain no amounts as they are equal. In contrast, the number of electrons in an ion is unequal, whereas the number of protons is equal, which means that they contain either a negative or positive charge. Examples of ions include sodium, potassium, chloride, calcium, etc.
Ions are of two types: either with a positive or negative charge. These are called anions and cations.
What is a Cation?
A cation is from an ionic family that contains a positive charge which means that it has more positively charged particles. Cations are formed when an atom loses one or more electrons. This loss of electrons results in a more significant number of protons. Because the more electrons will be lost, the more protons will be left. That is why the amount of proton in a cation is greater than the number of electrons. When an atom loses an electron, a positively charged particle is gained due to a smaller number of electrons as compared to protons. A “+” sign denotes it. The charge of an ion is symbolized after the chemical name, such as Zn2+ or Ag+.
Examples of cations include hydrogen, Calcium, Potassium, sodium, etc.
Properties of Cations
- Most of the cations are produced through metal atoms.
- Cations have electron deficiency as they contain a more significant number of protons than electrons. Hence cations are positive charge ions.
- Cations have one less orbit. Therefore cations have a smaller radius.
- When a cation interacts with a solvent in the form of a liquid, it produces solvated ions.
- Cations exist in both solid and liquid states.
- Cations react with anions to produce neutral molecules.
What are Anions?
Anions from an ionic family contain a negative charge which means that it has more negatively charged particles or a more significant number of electrons. It is bigger than a cation as it includes an extra number of electrons. The more the number of electrons, the greater the anion will be. Also, the more electrons are gained, the total charge will become negative. In other words, anions are formed when nonmetals gain more electrons. The charge of an ion is symbolized after the chemical name, for example, Cl– or OH–. The “-” sign denotes anions.
Examples of anions include Chloride, Hydroxide, Iodide, etc.
Properties of Anions
- Most of the anions are produced through non-metals such as Sulphate ions.
- They are negatively charged ions as they contain more electrons than neutrons.
- Anions contain more electrons; therefore, they have a bigger radius.
- Anions are more significant than solids.
- Anions exist in both liquid and solid states.
- Anions react with cations to produce neutral molecules.
- When anions interact with solvents in a liquid state, they produce solvated ions.
Difference Between Anions and Cations
Definition
Cation
A cation results when an atom or group of atoms generates more positive electric-charged particles or protons.
Anion
An anion forms when an atom or group generates more negative electric charged particles or electrons.
Origin of Word
Cation
The word cation was used by Faraday and Whewell but was taken from a Greek word called ‘kat ion,’ which means “going down.”
Anion
While, on the other hand, the anion is originated from a Greek word that means “going up.” Faraday and Whewell also used it.
Attraction
Cation
A large number of protons in a cation result in an increase in attraction force, leading to a decrease in the size of a radius.
Anion
However, the smaller number of protons in anion results in a decrease in attractive forces, increasing the size of a radius.
Reactions
Cation
When cations react with anions, it results in the formation of neutral molecules.
Anion
At the same time, when anions react with cations, it results in the formation of neutral molecules.
Periodic Table
Cation
When hydrogen produces hydron compounds, i.e., H2O, it represents a cation.
Anion
Contrarily, when hydrogen gains or loses electrons, it produces hydride compounds, i.e., ZnH2, representing an anion.
Type of Element
Cation
Elements like alkali and alkaline earth metals from the periodic table always produce cations.
Anion
But, elements like halogens always produce anions. Nonmetals like oxygen, sulfur, and carbon form anions.
Size
Cation
Cations are always smaller than neutral atoms as they contain fewer electrons.
Anion
At the same time, anions are always more extensive than neutral atoms as they contain more electrons.
Electric Charge
Cation
A cation has either one positive charge or multiple positive charges.
Anion
Alternatively, an anion has either one negative charge or multiple negative charges.
No of Electrons
Cation
In cations, the number of protons exceeds the number of electrons.
Anion
On the other hand, the number of electrons exceeds the number of protons in anions.
The Bottom Line
Anions and cations help us understand the physical and chemical properties of conductive substances around us. The charge difference in substances drives chemical reactions essential for physiological functions in the body and around us. Cations are positively charged ions while anions are negatively charged ions. One of the most significant differences between cations and anions is that the cathode attracts cations whereas the anode attracts anions. Sodium chloride contains sodium as the cation and chloride as the anion.
FAQs
What are cations and anions with an example?
Cations are positively charged due to a lack of electrons. Alternatively, anions are negatively charged as they have a higher number of electrons. Hydroxide ions are anions while magnesium ions are cations.
Is oxygen a cation or an anion?
Oxygen is a neutral pure substance with the formula O2. It becomes a positively charged ion (cation) on losing electrons and an anion on gaining electrons. It is represented as O2+ and O2- respectively.
Is silver a cation or an anion?
Silver is a neutral metal in its pure form and becomes Ag1+ on losing electrons. It allows the silver to conduct electricity.

Hello, I would like to introduce myself to you! I am Chelsea Rogers, an experienced blog writer for science articles, holding an MPhil degree. My enthusiasm to grab the best knowledge, let it relate to botany, zoology, or any other science branch. Read my articles & let me wait for your words s in the comment section.