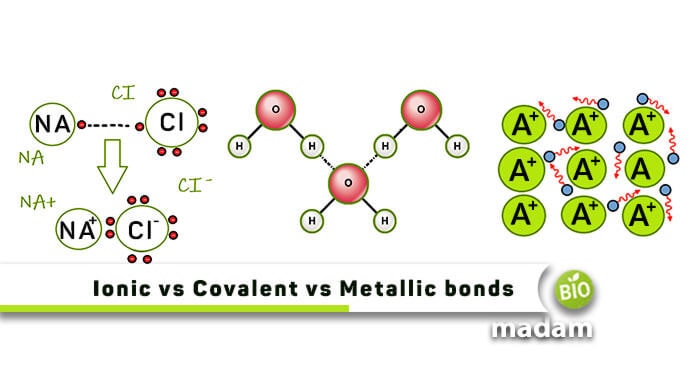
A chemical bond is the physical phenomenon and interaction of chemical substances held together by the attraction of atoms to each other.
This association helps form molecules, compounds, ions, crystals, etc., via sharing and exchanging electrons or electrostatic forces.
There are different types of chemical bonding, including covalent bonds, ionic bonds, metallic bonds, hydrogen bonds, etc. Covalent and ionic bonds are the main types of chemical bonds.
Covalent Bonds
It is also called a molecular bond, the mutual sharing of two or more electrons between two atoms. These participating electron pairs are known as shared pairs or bonding pairs, and shared electrons located in the space between the two nuclei are called bonding electrons. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
The interacting nature of atoms depends on their relative electronegativity (an atom’s ability to attract another atom or bonding pair). When the difference between the electronegativities of two atoms is too tiny for an electron transfer to form ions, it creates a covalent bond. These atoms have very high ionization energy.
The atoms share their electrons to obtain the octet configuration in their valence shell. It usually contains the energy of about ~80 kilocalories per mole (kcal/mol). Covalent bonds rarely break after formation.
Furthermore, they can be between the two non-metals or between two same elements. The covalent bonds within the molecules are powerful, and such interactions are highly directional, depending on the orbital overlap.
Properties of Covalent Bond
A covalent compound contains the following properties:
- In the physical state, they may exist as solids, liquids, or gases.
- Covalent molecules have definite shapes.
- They are usually soft and waxy by nature. This is because of the presence of a cloud of electrons between each layer of carbon atoms.
- They are readily soluble in non-polar solvents and insoluble in polar solvents.
- The compounds containing covalent bonds are non-conductors of electrical charge or have very low conductivity due to the absence of charged ions or free electrons. Graphite is an exception because of the presence of cloud of electrons.
- They are bad conductors of heat. Their molecules lack free electrons and that obstructs the flow of heat energy.
- They are very low or nonmalleable or non-ductile. Smaller covalent compounds with weak bonds are frequently soft and malleable
- Covalent compounds have low boiling points. This can be attributed to their weak force of attraction between the various bonded atoms.
Examples
HCl, H2O, PCl5, etc., are examples of covalent bonds.
Types of Covalent Bonds
The following types of covalent bonds based on electronegativity play a vital role in determining the different types of covalent bonding.
- Polar covalent bond
- Non polar covalent bond
Polar Covalent Bond
It is formed between two nonmetal atoms having different electronegativities and share their electrons (unequal sharing of electrons) in a covalent bond.
A part of the electron density of the bonding electron pair is closer to one of the bound nuclei, creating partially positive and negative atomic centers with the magnitude of the charge transfer. It depends on the relative electronegativities of the two atoms. The electrons cloud will shift to that atom that has high electronegativity.
These compounds can exist as solids due to the greater force of interactions and have high melting and boiling points. They are soluble in polar compounds such as water.
Examples of Polar-Covalent Bonding are:
- Bonds between hydrogen and other elements such as oxygen (H2O).
- The bond between hydrogen and other atoms such as Cl (HCl)and F (HF)
Non Polar Covalent Bond
It is formed between two atoms with the same electronegativities, sharing equal electrons in a covalent compound. The difference in electronegativity is mostly negligible in non-polar covalent bonds.
They exist in gas form but rarely in liquid form and are very soft. They have low boiling and melting points and are soluble in non-polar solvents.
Examples:
H2, N2, O2, Cl2, etc. are examples of non-polar covalent bond
Other Types of Covalent Bonds
There are different types of covalent bonds based on the number of shared electrons pairs.
- Single covalent bond
- Double covalent bond
- Triple covalent bond
Single Covalent Bond
In a single covalent bond, only one pair of electrons is shared between two atoms. It is represented by one dash (-). It is a weaker bond as compared to the double and triple bond. It has less density. It is the most stable bond.
Example: The bond between hydrogen and hydrogen (H-H) is an example of a single covalent bond. Another example of a covalent bond is F2, HCl, etc.
Double Covalent Bond
When two pairs of electrons are shared between the two atoms, the bond formed is called a double bond. It is represented by one dash (=). Moreover, it generates by one Pi bond and one sigma bond. It is a strong bond compared to a single one but with lesser stability.
Example: The bond between two oxygen atoms (O=O) is an example of a double covalent bond. Other examples are CO2, C2H4, acetone, ozone, etc.
Triple Covalent Bond
In a triple covalent bond, three pairs of electrons are shared between two atoms. It is the least stable than general types of covalent bonds. It is represented by three dashes (≡).
Example: N≡N is an example of a triple covalent bond.
Ionic Bonds
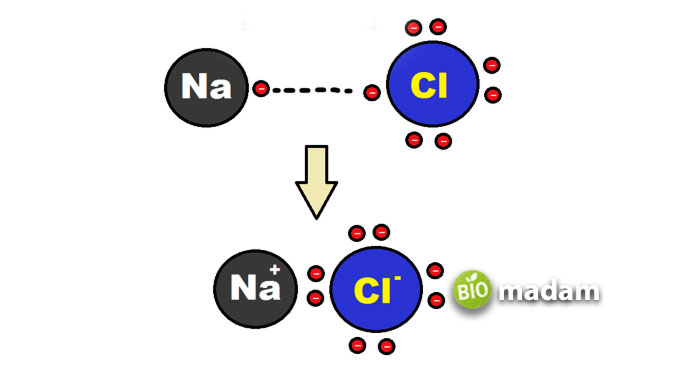
An ionic bond is also called an electron covalent bond, formed by the complete transfer of some electrons (outermost orbital) from one atom to another. Electron transfer produces negative ions called anions and positive ions called cations.
These ions attract each other and are developed by electrostatic bonding with opposite electric charges. They form between atoms with significant differences in electronegativity. In forming the ionic bond, the octet is achieved by gaining or losing electrons from the atoms. In this bond, ions are arranged in a three-dimensional array of crystals, and they dissociate into ions in solution.
It is a common feature of the inorganic compounds and the salts of organic molecules. Ionic bonds depend on the atom’s radius, where the larger the radius, the more likely the compound will have ionic bonding. It is mainly formed between a metal and a nonmetal atom.
Properties of Ionic Bonds
There are the following properties of ionic bonds.
- They exist as solid-state.
- Ionic bonds are hard because of their crystalline nature and also have high melting and boiling points.
- This type of bond has high bond energy than the metallic bond.
- They are non-malleable and non-ductile.
- As compared to other bonds, this is considered an insulator of electricity but is a conductor in molten state due to the presence of ions which act as charge carriers.
- Ionic bond dissociates into an ion soluble in water.
- This bond is the strongest bond among others and is highly brittle.
Following are some examples of ionic bonds.
KCl (Potassium Chloride)), CsF (Cesium Fluoride), BeS (Beryllium Sulfide), NaCl (Sodium chloride), etc.
Metallic Bond
This chemical bond holds atoms together among the metals and shares the free electrons among the lattice of cations as it occurred in metals or alloys, hence called metallic bonds. It is different from the covalent bond because ionization energy for electrons occupying the outer orbitals of the metallic elements is much smaller.
When the hybridization is absent, s-orbital (allowing overlapping with up to 12 further s-orbitals of the surrounding atoms) leads to the formation of a ‘metallic’ bond (non-directional), whereas d-orbital leads to the formation of a ‘covalent’ bond (directional).
Different factors affecting the strength of a metallic bond include
- the total number of delocalized electrons
- the magnitude of positive charge held by the metal cation
- the ionic radius of the cation.
Alloy is formed through metallic bonding. Examples of alloys are brass (Cu and Zn) and steel (C and Fe). Other metallic bonds are iron, cobalt, calcium, magnesium, silver, gold, etc.
Properties of Metallic Bonds
Following are the properties of metallic bond-containing compounds
- Metallic bonds are usually in a solid state.
- They are hard in nature and have no definite shape.
- If we see its solubility in non-polar and polar solvents, they are insoluble.
- Most metals are excellent electrical conductors because the electrons are free to move and carry the charge.
- Metallic bonds are malleable and ductile with high melting and boiling points as well as low volatility.
Ionic, Covalent, and Metallic Bonds – Primary Differences
| Covalent bond | Ionic bond | Metallic bond |
|---|---|---|
| Sharing of electrons between two atoms | Complete transfer of electrons | Sharing of electrons between metals lattice |
| Present among non-metals | Present among metals & non-metals | Present among the metals |
| Strong bond than the metallic bond | Strongest bond | Weak bond than others |
| Exist in solid, liquid, and gas state | Exits as solids | Exits in solid-state |
| Bond is directional | Bond is nondirectional | Bond is nondirectional |
| E.N. for polar covalent is 0.5-1.7 and for non-polar is ˂ 0.5. | ˃ 0.7 is electronegativity | E.N. is not required. |

Hello, I would like to introduce myself to you! I am Chelsea Rogers, an experienced blog writer for science articles, holding an MPhil degree. My enthusiasm to grab the best knowledge, let it relate to botany, zoology, or any other science branch. Read my articles & let me wait for your words s in the comment section.