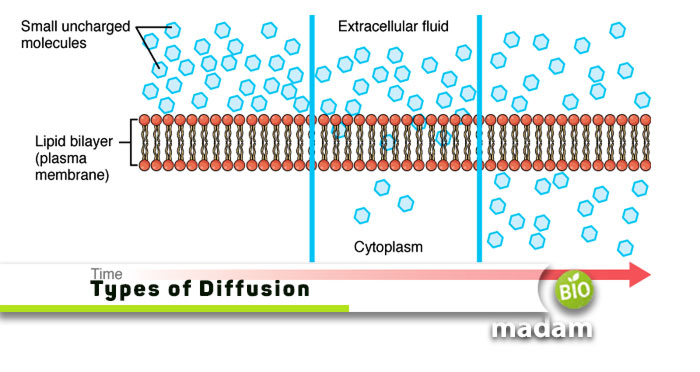
Our biome and ecosystem comprise different living and nonliving components. Each type of organism has unique anatomy and physiological processes occurring within the body. Similarly, some processes occur in the surroundings ensuring the exchange of material to sustain the environment. Diffusion is one such process that takes place in different types of ecosystems and within the body.
Let’s tell you about the different types of diffusion.
What is Diffusion?
Diffusion is the movement of molecules from an area of higher concentration to areas of lower concentration under the concentration gradient.
The molecules move from an area of higher concentration to a lower one because of the difference in concentrations. It can takes place in solutions as well as suspensions, on the principle of random motion. The process of diffusion is essential because it occurs in all living organisms, and it helps in the movement of the cells. This motion of molecules continues until the molecules of higher concentration and lower concentration become equal.
Factors Affecting Diffusion
Diffusion is a dependent process that majorly depends on the concentration difference between the liquid or aqueous solutions. It also depends on other factors like distance and temperature. Let’s tell you about all of them:
Concentration Gradient
The concentration gradient affects the rate of diffusion significantly. The greater the concentration gradient, the faster is the rate of diffusion. For example, when there is a very high concentration of oxygen and a very low-level concentration of blood, diffusion will occur faster than usual. But when there is an equal concentration level of both in the above-mentioned condition, then the rate of diffusion will be pretty low.
Temperature
Apart from the concentration gradient, temperature also plays a major role in the diffusion rate. When the temperature in one or both solutions rises, the particles gain more kinetic energy. Eventually, they move faster and lead to a higher rate of diffusion. So, the diffusion rate depends on the rise and fall of temperature as well.
There is a directly proportional relationship between temperature and the rate of diffusion. As the temperature increases, the rate of diffusion will also increase.
Distance
Distance is one of the main elements that play a significant role in diffusion. The farther the distance between the substances or mixtures, the slower the diffusion rate will be. Similarly, molecules closer to each other increase the diffusion rate. The change in the diffusion rate occurs due to the time taken for the molecules to travel to the other side. Molecules take more time to move across a longer distance and vice versa.
Surface Area
Surface area is directly proportional to the rate of diffusion. As the surface area increases, the diffusion rate will also increase. This happens due to the space around the molecules. The larger area corresponds to an increasing volume at a higher rate than the surface area. Thus, the surface area to volume ratio decreases, leading to an increase in diffusion rate.
Types of Diffusion
Diffusion exists everywhere in every field, such as chemistry, physics, biology, etc. It can be divided into two main types: simple diffusion and facilitated diffusion. These two types of diffusion differ regarding the mechanism and how the element’s or compound’s molecules move. The main difference between simple and facilitated diffusion is their movement regarding the concentration gradient. Simple diffusion moves along the concentration gradient. Contrarily, facilitated diffusion may occur in the opposite direction as well. Primarily, facilitated diffusion requires facilitation by other factors such as proteins.
Here’s all you need to know about simple and facilitated diffusion.
What is Simple Diffusion?
Simple diffusion is also known as free diffusion, as it moves freely from one region to another without needing external molecules. It does not require energy to facilitate the movement of molecules from higher concentration to lower concentration. Chemical bonds between solutes and water contribute to the process of simple diffusion.
Tiny molecules pass through the cell membrane between the phospholipids without any hurdles. This kind of diffusion involving the movement of water across the membrane is also referred to as osmosis. Read the differences between diffusion and osmosis here.
Mechanism of Action
Concentration gradient and kinetic energy fuel simple diffusion instead of using ATP to move the molecules. The molecules collide due to the constant random motion occurring in liquids. It takes place when the given region has larger spaces. The molecular movement between both areas continues until equilibrium is reached.
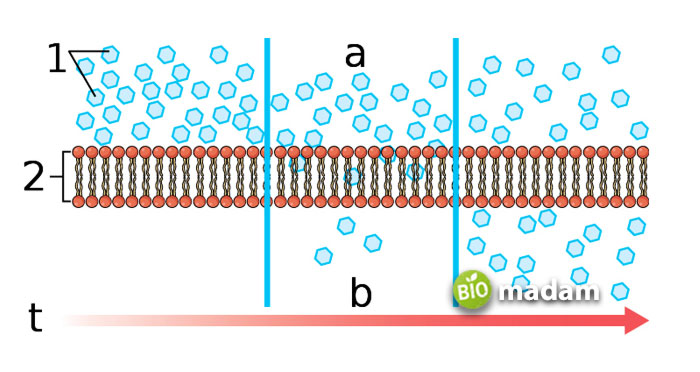
Example of Simple Diffusion
One of the most common examples of simple diffusion is the movement of water, oxygen, and smaller molecules across the cell membrane in microorganisms like archaea and bacteria. As they do not have any special organelles to transport substances across the cell membrane, diffusion helps them intake and get rid of unwanted substances.
What is Facilitated Diffusion?
Facilitated diffusion refers to the movement of molecules from an area of higher concentration to an area of lower concentration across the cell membrane. This diffusion type requires help moving the substances across the cell membranes. This help is provided to the molecules by proteins that facilitate the movement of substances. Chemical energy is not directly utilized in facilitated diffusion.
Mechanism of Action
As mentioned above, the process is enabled by the help of transmembrane proteins in the cell. These plasma membrane proteins help in the transport of outside material, and are particularly of two types: channel proteins and carrier proteins. Channel proteins help enter and exit cellular substances while carrier proteins carry the molecules. Open channel and gated channel proteins work together to only welcome or remove the required molecules. On the contrary, carrier proteins are present on the animal cell membrane and release the molecules after changing their conformation. Temperature and saturation impact the function of carrier proteins.
Example of Facilitated Diffusion
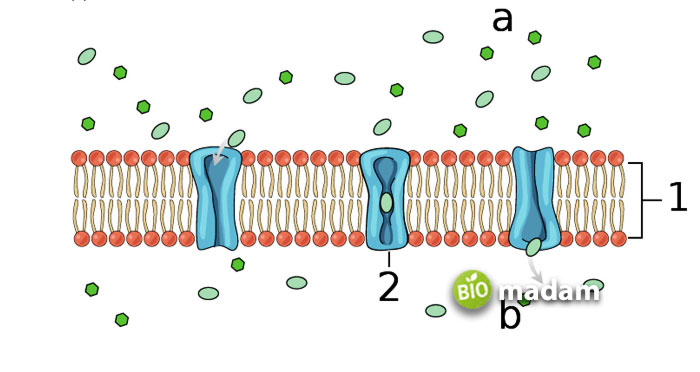
Ion channels are the most well-known example of facilitated diffusion, using ionic pumps for the movement of molecules. They act to maintain the sodium-potassium pump in cells. Excess sodium ions in the extracellular space lead to resting potential. A slight voltage change pushes the sodium ion channels to open, allowing sodium ions to enter the cells. Subsequently, potassium moves out of the cell.
Diffusion in Solids, Liquids, and Gasses
The basic mechanism of action of diffusion is the same in the branches of biology, chemistry, and physics. However, it contributes to a specific function in every domain.
Understanding the difference between diffusion in different states of matter comes down to the available space. The suspended molecules in gasses and liquids hit each other, resulting in random motion. The movement of the molecules occurs due to the collision of molecules with each other. This process is also known as Brownian motion. As the number of molecules increases, they become tightly packed with each other. When the space is provided, molecules will move from a region of higher concentration to a lower concentration. Lighter and smaller molecules spread more quickly as compared to heavier molecules. That is why gas molecules diffuse more quicker than molecules of solids or liquids.
The Bottom Line
Diffusion is a widely occurring phenomenon around us and is vital to life. It enables the transport of molecules across the cell membrane to take in important substances and get rid of unwanted molecules. Besides its activity in different types of eukaryotic cells, it also contributes to the sustenance of bacteria and archaea. Water and smaller nutrient molecules move in and out of the cell through simple diffusion. On the other hand, facilitated diffusion plays a major role in glucose and amino acid transport, ion transport, and gas exchange in animals.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.