Recently updated on May 2nd, 2023 at 09:34 am
Boiled water and distilled water are often confused as they are thought to be free of impurities. While it is true for distilled water, the same cannot be said about boiled water. Most people stop at a common question: is boiled water the same as distilled water? The answer is “no.” Although they both include the liquid-gas states of water, they are different. Distilled water is widely used in college lab experiments for chemical analysis. On the other hand, boiling water is often utilized in households for different purposes.
So, boiling water and distilled water are not the same.
Let’s tell you the differences between boiling and distilled water.
What is Boiling Water?
Boiling is a physical property of any matter and refers to heating a substance long enough to reach its boiling point. The boiling point is the temperature at which a liquid or an aqueous solution turns into vapors.
Water has a boiling point of 100°C which means it boils when it reaches 100°C. Boiling is an effective method to remove microscopic parasites and bacteria from water. Bacteria do not survive above 45°C. Thus, boiling is an effective way to remove these impurities from water.
So, boiling water does not contain germs like bacteria but still has impurities, including mineral traces. This water is suitable for sanitization. However, studying pure substances or mixtures in laboratories is not a preferred choice. The minerals in the water may interfere with your observations.
Process of Boiling
The boiling process is simple compared to distillation, but what happens to water molecules during the boiling process? Let us tell you that below:
You only need a burner, utensil or beaker, and water to start the boiling process. Take water in a utensil or a beaker where you want to heat it. Place the beaker on a tripod stand and then onto the burner. Turn on the burner to provide heat to the water. The water will start heating and gradually move towards the boiling point. The liquid will turn into bubbles when it reaches the boiling point.
Here are the four stages of boiling:
- Slow Simmer: Slow simmering is the first step of the boiling process when the heat is low, and there is little movement in the container.
- Simmer: You will start spotting small bubbles at the bottom of the container when the water starts simmering.
- Rapid Simmer: The water starts simmering rapidly as it absorbs more heat and will boil soon.
- Boiling: Water absorbs more heat and starts boiling when the temperature reaches 100°C.

Applications of Boiling Water
Boiled water can be used for drinking as it is free from microorganisms and prevents water-borne bacterial infections. It is often used for washing stains compared to room-temperature water. Boiling water is a disinfectant for cutting boards, especially when you want to cut vegetables or fruits after using it for meat.
What is Distilled Water?
Distilled water is free from all impurities, including antigens, pathogens, minerals, etc. Distilled water is prepared through an extensive process that removes anything besides water molecules from the water. The water droplets formed on steaming are basically distilled water. They do not contain any particles.
Distilled water, also known as pure water, goes through the ion exchanger, electrodialysis, and reverse osmosis besides distillation. Distilled water is used in different chemical reactions and analytical techniques in laboratories. Thus, it is packed into a sealed container. This water is transparent, colorless, and safe to drink. However, drinking water is not the right choice as it is void of minerals.
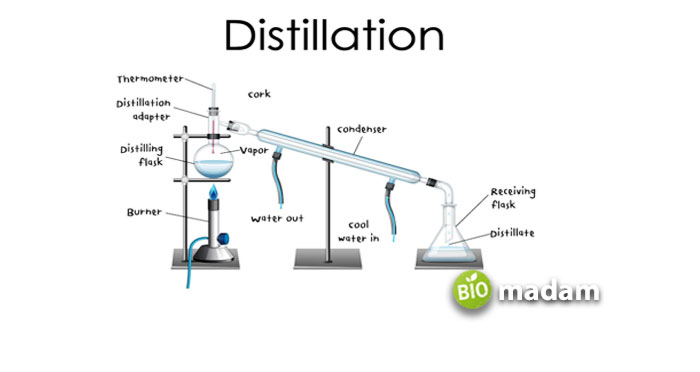
Process of Distillation
The distillation process requires distillers that boil and condense water in a single unit. The process takes more time than boiling but does not require multiple instruments. You can carry out distillation within the distiller until distilled water is produced. Here is the simple process of distillation:
Step 1: Add water to the boiling chamber and turn on the switch to heat water to the boiling point.
Step 2: This process leads to the formation of steam that passes through a passage.
Step 3: The vapors cool down on their way and convert into water droplets.
Step 4: These water droplets are collected in a clean container to be sealed later.
The carbon filter stops the movement of minerals or other impurities trying to make their way into the water droplets. Though the process is quite similar to boiling, condensing vapors into droplets individually takes a long time. A lab or home water distiller may take up to 6 hours to produce a gallon of distilled water.
Applications of Distilled Water
The most common application of distilled water is in laboratories in chemical experiments and pharmaceuticals. They are also utilized in other industries, like cosmetic industries, where the water used in preparation must be free from particles. Most fish tanks and aquariums also contain distilled water to create a suitable environment for the fish.
Differences Between Boiling and Distilled Water
Components
Boiling water removes all the germs, including viruses, bacteria, protozoa, and other parasites, from the water, but it might not get rid of trace minerals. However, distillation eliminates all forms of particles and impurities from water to make it “pure water.”
Drinkability
Both boiling and distilled water are suitable for drinking. However, you must not drink distilled water for long as it can lead to mineral deficiencies.
Utilization in Aseptic Preparations
Pharmaceutical and cosmetic industries utilize distilled water to manufacture aseptic preparations and prevent contamination. It can also be used in analysis and research in chemical industries.
The Bottom Line
Many people stay confused about boiled water and distilled water throughout their life, but it should be addressed. While boiled water is free from microorganisms, it still contains trace minerals and other impurities. But distilled water does not contain minerals either and is “pure water.” Boiled water has various home uses, like drinking, cleaning stains, and disinfecting utensils. Meanwhile, manufacturing industries use distilled water to ensure purity.
FAQs
Can I use boiled water instead of distilled?
Distilled water is not suitable for manufacturing industries as it contains trace elements. Distillation removes all kinds of impurities from water, making it suitable for use in research and manufacturing.
How do I make distilled water at home?
You can use a distiller to make distilled water at home. Boil the water to turn it into vapors, and then let them condense into water droplets. This will leave the minerals and impurities within the container and give you distilled water in a different container.
Is bottled water distilled water?
Bottled or mineral water differs from distilled water as it is not entirely pure. Bottled water contains essential minerals in the required quantity to maintain normal body physiology.

Meet me; I am Paulina Zaniewska, who’s more hooked on providing the best health blog. I’ve always been so determined to compete as a nutritionist, and here I am, done with a Master’s in food technology. My brilliant performance throughout encouraged me to help people.

