Recently updated on October 4th, 2023 at 08:21 am
Chemistry deals with the structure, composition, and properties of substances. Every substance found in the world, whether it is natural or artificial, is composed of atoms. Atoms are considered to be the building blocks of any chemical substance. These atoms make up an element with a different number of electrons in their valence shell. These elements gain or lose electrons to stabilize themselves. Once they gain or lose electrons, they become charged species called ions. These ions are of two types called anions and cations.
Metals constitute the main part of the periodic table. There are almost 93 metals found in the periodic table. Now, the most common question coming to our minds is ”do metals form anions or cations?”
The short and simplest answer is ”metals only form cations.” But how does the formation of cations take place?
Keep reading the article below to learn more about cations and anions, their properties, their differences, and why they form cations.
What are Metals?
Metals are natural inorganic substances found inside the earth’s crust. Most of the metals found naturally are shiny and lustrous. They have high malleability, high thermal and electrical conductivity, and high reflectivity of light. The most abundant elements found in the Earth’s crust are aluminum, sodium, calcium, iron, potassium, and magnesium. Some other metals that exist in the form of ores are called metal ores. These include copper, gold, silver, and platinum. If we talk about its physical state, it has many as sodium and potassium can be cut by a knife, whereas mercury is a liquid and iron is solid at room temperature.
What are Cations and Anions?
Atoms are said to be neutral when they have equal positive and negative charges. If they lose this balance they get charged and form ions. Cations are a type of charged species having more protons than electrons in their valence shell. Just like that, anions are the type of charged species having more electrons than protons in their valence shell. To stabilize themselves, they gain electrons and get negatively charged. They gain these electrons by pulling them from atoms that have a weaker affinity for them.
Examples
Sodium (Na) forms cations after losing one electron from its valence shell to stabilize itself. After losing the electron it becomes ”Na+.’’ Similarly, chlorine (Cl) has seven electrons in its valence shell, it gains one electron to form an anion. Then it is represented as ‘’Cl–.”
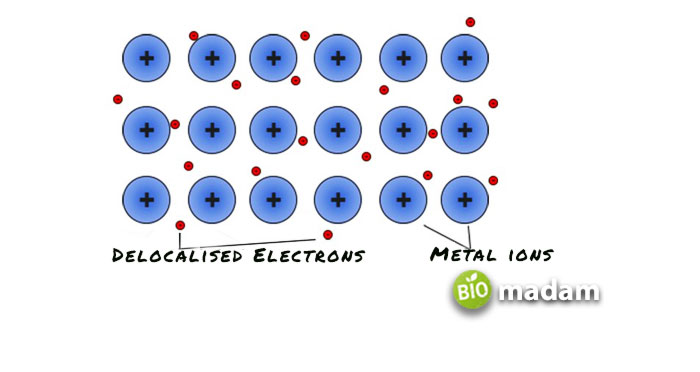
Do Metals Form Anions or Cations?
Metals tend to form cations as they hold their outermost electrons loosely. They are likely to lose these electrons and get a positive charge. Whereas non-metals tend to attract electrons more strongly and thus get a negative charge after gaining electrons. These positively and negatively charged ions combine to form a stable ionic compound with no overall net charge.
For example, sodium loses one electron to form Na+ and Cl gains an electron to form Cl–. The Na+ and Cl– combine to form the ionic compound NaCl.
Difference Between Cations and Anions
The following table contains some major differences between cations and anions.
| Property | Cations | Anions |
| Charge | Positively Charged | Negatively Charged |
| Formed By | Metal Atoms | Non-Metal Atoms |
| Attracted To | Cathode | Anode |
| Electrostatic Interactions | Interactions with anions to form ionic compounds | Interactions with cations to form ionic compounds |
| Size | Smaller than parent atom | Larger than parent atom |
| Example | Na+, K+, Ca++ | Cl–, Br–, I– |
How to Predict if a Substance Forms Anion or Cation?
Whether an atom forms a cation or an anion depends on its position in the periodic table. Alkalis and alkali earth metals always tend to form cations whereas halogens always tend to form anions. Most of the other non-metals also tend to form anions. However, some elements have the capability of forming both anions or cations depending on the conditions. The group 18 elements called the noble gases do not form any ions. They are considered unreactive.
How to Write Chemical Formulas?
The correct way of writing a chemical formula is to mention the cation first and then the anion. For example, sodium forms a cation, and chlorine forms the anion then the chemical formula for sodium chloride is written as ”NaCl.”
Similarly, the charge on the cations or anions is mentioned after the symbol of the element in the form of a superscript. For example, Mg+2 and Ca+2.
The Bottom Line
Atoms make up the entire universe as every substance consists of atoms. These atoms tend to lose or gain electrons to stabilize themselves. Once they gain or lose an electron, they get charged and form ions called either cations or anions. Metals form cations by losing their electrons to stabilize themselves. While the non-metals form anions to stabilize themselves. We hope that after reading this article the concept of cations and anions and how metals form cations become clear.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.