Cations and anions are important terms when studying chemical reactions. They allow us to understand how a particular element, molecule, or a compound acts in a chemical reaction. The charged form of an atom or molecule is known as its ion. Positive ions are called cations, while negative ions are anions, but the question arises how these ions are formed? Today, let’s discuss the formation of cations in this article.
What are Cations?
Cations are positively charged ions liberated due to one reason or the other. Ions achieve a positive charge when the atom or molecule loses an electron. Thus, the number of protons becomes higher than electrons, giving it a positive charge. Any kind of chemical substance, including pure substances and mixtures, may have cations.
The electrons are present outside the nucleus of the atom, while the protons and neutrons are within the nucleus. The electrons or negative charges surround the nucleus in the form of a cloud. The positively charged protons have a location near the neutrons in the atom’s nucleus. Neutrons play a vital role in nuclear physics, yet they have no impact on the charge of the atoms they inhabit, making them irrelevant to ionization. As we explained above, finding the difference between the total numbers of protons and electrons yields the net charge.
In their unaltered state, an element’s atom will contain an even amount of electrons and protons, despite their opposed charges. Since the charges of the protons and electrons cancel each other out, neutral elements have no net electric charge.
Thus, the number of electrons increases when an atom receives an electron. The atom obtains a negative charge and forms an anion. Similarly, the loss of electrons leads to positively charged cations forming.
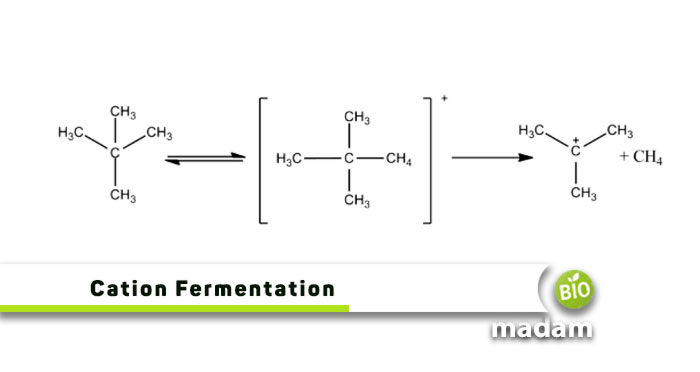
The Process of Cations Formation
After a neutral atom loses an electron, it becomes a cation. As a result of the way their electrons orbit the nucleus, metals are particularly vulnerable to electron loss. The orbits that electrons fill around atoms’ nuclei correspond to distinct energy levels. An electron in a high-energy orbital is further from the nucleus than one in a lower-energy orbital.
Stable atoms have extremely overpopulated energy levels, but those with only a few electrons are more likely to undergo electron loss. The electrons at the highest energy levels “shield” the nucleus from a lot of the positive charge.
This results in weaker chemical bonds between the nucleus and the outer electrons. Cations can form via various chemical reactions like electron transfer reactions or ionization.
As a result of a chemical reaction, an atom may gain or lose electrons, resulting in a cation or anion. NaCl, or table salt, results when sodium gives an electron to chlorine, turning it into a cation (Na+). During an electron transfer reaction, one atom can lose an electron to another atom or group of atoms. A copper ion reacts with zinc metal to form zinc cations (Zn2+).
Ionization
The process of formation of ions is called ionization. This leads to the creation of positive and negative ions.
One definition of ionization is the creation of a cation by stripping an electron from an atom or molecule. Several methods can accomplish this, including applying heat, undergoing a chemical reaction, or exposing the material to radiation.
Example
A good example is the formation of sodium cations (Na+) at high temperatures, causing it to become ionized. A cathode, a negatively charged electrode, will draw cations, or positively charged ions, in its direction.
The process of ionization produces cations when electrons receive enough energy (through, for example, high-energy light) to escape the attractive force of a nucleus. Ionization energy refers to the amount of power needed for this process. A single electron requires the first ionization energy to ionize. The second represents the energy necessary to ionize two electrons, and so on.
Significance of Cations
In the periodic table, Group I, Group II, and Groups III–XII metals are common starting points for making cations. Cations are scattered everywhere in the natural world. Some of the most significant applications of cations in our surroundings include:
- The cation acetylcholine is a neurotransmitter, as well as serves as a hormone, responsible for the transmission of nerve impulses.
- Lithium batteries can store and release electrical energy thanks to the interaction of cations and anions. Under their charge, cations migrate from the anode to the cathode within the battery.
- Water homeostasis in cells: Potassium cations are often found within animal cells. Sodium cations (Na+) are widespread in extracellular environments like blood and plasma. The sodium-potassium pump is a mechanism present in many animal cells that controls this ion balance.
The Bottom Line
Cations are an essential aspect of chemistry in our daily lives. They are positively charged molecules that lose their electrons. Cations are formed through ionization when molecules react and take away electrons from each other. The charges move from the anode to the cathode, contributing to various functions. They are critical to various cellular functions, including substrate level and oxidative phosphorylation.
FAQs
How are anions formed?
Anions are negatively charged ions that obtain the charge when they take electrons from other atoms. They are typically produced by ionization during chemical reactions.
What elements create cations?
The elements of Group 1A and 2A of the periodic table always produce cations on ionizations. They are alkali metals and alkaline earth metals that lose electrons and obtain a positive charge.
How are cations formed by oxidation?
Oxidation is the loss of electrons that leads to a positive charge on that atom or molecule. Non-metals usually produce cations through oxidations, while metals become anions through reduction.
How do you identify a cation?
Cations can be identified through the flame test. A sample of the material under analysis is placed on an unreactive metal wire on a flame. The color of the flame lets you identify the type of metal.
What are the 5 major cations?
The five major cations around us include Calcium Ca+, Magnesium Mg+, Sodium Na+, Potassium K+, Aluminum Al+.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.