Recently updated on October 30th, 2023 at 09:38 am
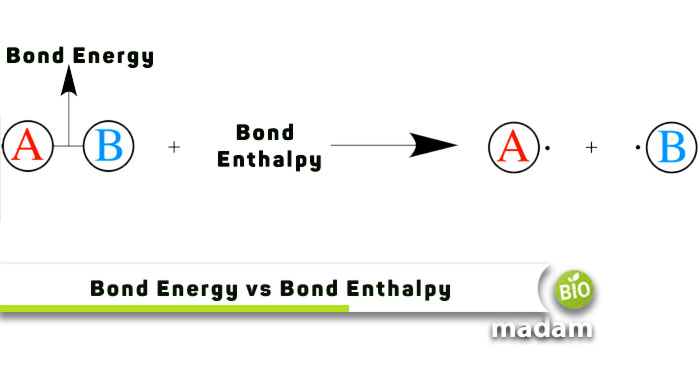
The formation and breakdown of different bonds like ionic, covalent, and metallic bonds within molecules require energy. The energy changes are explained by bond energy and bond enthalpy. The main difference between bond energy and bond enthalpy is the bond characteristics they explain. Bond energy is the measure of the bond’s strength, while bond enthalpy is consumed or released on dissociation or formation of a bond. Bond enthalpy is generally high as bond formation, or breaking requires a lot of energy. Keep reading to learn about bond energy and bond enthalpy in detail.
Comparison Table
| Factors | Bond Energy | Bond Enthalpy |
| Definition | Bond strength | Bond dissociation |
| Symbol | denoted by E | denoted by H |
| Function | Base for bond formation | Bond breakage |
| Consistency | Inconsistent | Consistent |
| Bond Formation | Atom production | Free radical production |
What is Bond Energy?
An approximate measure of the energy needed to split atoms in a molecular bond into their parts is the bond energy. Bond energy acts as a measure of the strength of a chemical bond. It indicates how likely a bonded pair of atoms will break apart in the presence of energy perturbations.
Another way to look at this concept is as a quantitative indicator of the relative improvement in stability between the bound and unbound states of two atoms. In the potential energy curve, bond energy is equivalent to the depth of the “well” or the work required to separate two atoms.
The “E” represents the energy of a bond. kJ/mol is the unit of measure.
The bond order is the number of chemical bonds between atoms in a molecule or a compound. Shorter bond lengths indicate stronger bonds, and more bonds between atoms mean stronger bonds. That’s why triple bonds are the quickest, followed by doubles, and finally, singles. Eventually, triple bonds are the strongest, double bonds are the following best, and single bonds are the weakest.
So, the bond strength between a pair of atoms is greater, and the bond length is shorter when the bond energy is higher. The two atoms are more tightly bound when the bond energy is high.
Example
The bond energy required to split one molecule of CH4 into a carbon atom and four hydrogen atoms equals the (C-H) carbon-hydrogen bond energy in methane (CH4) divided by four. The C-H bond in methane has a bond energy of 413 kJ/mol.
Alternatively, one mole of O-H bonds in a compound of water has a bond energy of 464 kJ/mol, meaning it takes 464 kJ of energy to break those bonds.
What is Bond Enthalpy?
In thermodynamic systems, enthalpy (H) denotes a measure of the total heat energy present. It is easy to calculate a reaction’s enthalpy change using the bond enthalpy, which is the energy needed for the reaction to occur. The unit of measurement is kJ/mol.
The term “change in enthalpy” refers to the overall energetic shift brought about by the process and is calculated by adding the enthalpies of all bonds broken and formed during the procedure.
The reaction can be either endothermic or exothermic, according to whether the enthalpy is positive or negative. Whenever the reaction’s change in enthalpy (H) is positive, it is endothermic; whenever it is negative, it is exothermic.
It takes an increase in enthalpy to dissolve a bond, while a decrease in enthalpy is needed to create one. Therefore, bond formation is an endothermic process, and bond breaking is an exothermic one.
Example
The bond enthalpy energy for the four C-H bonds in a methane molecule is 439 kJ/mol, 460 kJ/mol, 423 kJ/mol, and 339 kJ/mol, respectively.
Difference between Bond Energy and Bond Enthalpy
Definition
Bond Energy
Bond energy is the measure of the bond’s strength in a chemical bond. It is an average value.
Bond Enthalpy
The bond enthalpy, on the other hand, required to dissociate a bond in homolysis is a measure of the energy needed to break apart that bond.
Symbol
Bond Energy
The symbol for bond energy is an E.
Bond Enthalpy
Whereas, bond enthalpy in chemistry is represented by H.
Measure
Bond Energy
Bond energy is typically not as high as bond enthalpy.
Bond Enthalpy
But, breaking a bond requires an extraordinary amount of energy.
Outcome
Bond Energy
The atoms that serve as the basis for bond formation require a certain amount of energy, and bond energy can provide it.
Bond Enthalpy
On the contrary, the bond enthalpy breaks the bond and allows the formation of free radicals from the original atoms that formed the link.
Variation in Energy
Bond Energy
Each bond has a distinct amount of energy, which is not constant.
Bond Enthalpy
However, in the case of the enthalpy of the formation of bonds, the value remains consistent across all bonds.
Bond Formation
Bond Energy
Bond energy provides the driving force for the production of the atoms that are the building blocks of chemical bonds.
Bond Enthalpy
At the same time, it gives the necessary energy for forming free radicals from the original particles that developed that specific bond.
Examples
Bond Energy
Methane (CH4) has a C-H bond with an energy level of 414 kilojoule/mole.
Bond Enthalpy
Conversely, in the case of methane (CH4), the bond enthalpies for C-H bonds are as follows: 439 kilo-joule/mol, 460 kilojoule/mol, 423 kilojoule/mol, and 339 kilojoule/mol respectively.
The Bottom Line
Bond enthalpy and bond energy are important terms in chemistry and are often used interchangeably. While you may use these terms in place of each other in particular situations, they do not always represent each other. Bond energy refers to the bond’s strength in a chemical reaction whereas bond enthalpy explains the energy required to break down a metallic, ionic, or covalent bond. The bond enthalpy is typically higher than bond energy. Additionally, the bond energy might not remain constant while bond enthalpy remains consistent. It allows the researchers to understand the energy required to make changes through chemical changes.
FAQs
Is bond enthalpy always positive or negative?
When bond enthalpy refers to the energy required to break a chemical bond, the values are represented as positive as the reaction obtains energy.
What is the mean bond enthalpy?
The mean bond enthalpy refers to the average of different compounds to determine the bond enthalpy of a covalent bond in a molecule.
What increases bond enthalpy?
As the bond enthalpy is required to break bonds, a higher number of bonds means higher energy is required. Moreover, the repulsion between the atoms due to the number of lone pairs also increases the bond enthalpy.
Does bond enthalpy depend on temperature?
Yes, the temperature may impact the bond enthalpy. The bond enthalpy usually decreases with increasing temperature and vice versa.
Which bond is the strongest?
Ionic bonds are the strongest compared to covalent and hydrogen bonds. Thus, they take the highest amount of energy to dissociate.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.