No doubt, ionic and covalent bonds are different according to bonding formation, but they are similar in many ways. But, how are ionic and covalent bonds alike? Before we understand the similarities of ionic and covalent bonds, it is must to know what is an ionic and covalent bond.
What is an Ionic Bond?
An ionic bond is the attraction between two oppositely charged ions come near to each other and connect. Both ions attract one another and result in the loss and gain of the electrons from the outermost shell to complete the octet. Octet means acquiring 8 electrons in the outer shell.
Some ions get positive, and some get a negative charge. Ions that lose electrons get a positive charge and have more protons than electrons. Negatively charged ions gain electrons instead of losing and have more electrons than protons. In an ionic bond, complete loss or gain of electrons takes place between the ions.
Example of an Ionic Bond
Chemistry is filled with examples of ionic bonds. Salts like NaCl, NaF, KCl, etc., are the best examples.
What is a Covalent Bond?
The covalent bond is the sharing of electrons between the atoms and has the complete valence shell. The valency of the covalent bond, through the outermost shell, tells the need for electrons. It works differently than an ionic bond. The constituent atoms continue sharing the bond through their valence shells until an external force breaks them.
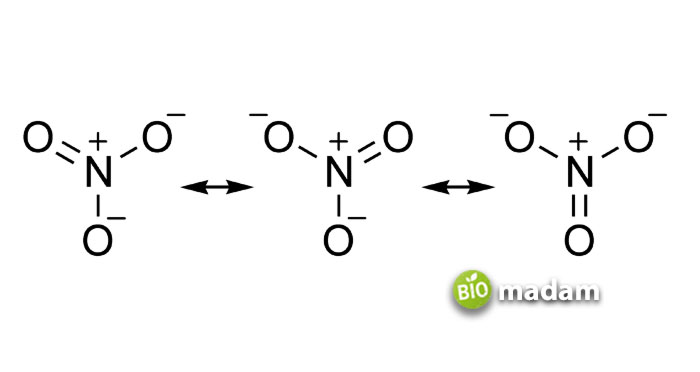
The bond can be polar or non-polar, depending on its electronegativity and other factors. But, whether its polar or non-polar, both have definite shape. Overall discussing the nature of covalent compounds, they have low melting and boiling points and are more flammable.
Example of a Covalent Bond
All the organic compounds, including long chains of hydrocarbons (methane, ethane, propane, etc.), oxygen molecules, and water are examples of covalent bonds.
Let’s take a look at the brief table between ionic and covalent bonds.
| Factors | Ionic Bond | Covalent Bond |
|---|---|---|
| Involvement of Electron Pairs | Present | Present |
| Electrostatic Forces | Present | Present |
| Type of Bonding | Through the transfer of Ions | By sharing electrons |
| Follow Octet Rule | Yes | Yes |
| Provides Stability | Yes | Yes |
| Type of Reaction | Exothermic | Exothermic |
| Solid State Compounds | Show Fixed Pattern | Show Fixed Pattern |
| Effect of Temperature | Changes Shape | Changes Shape |
| Effect of Pressure | Changes Shape | Changes Shape |
| Solubility | In Polar Compounds | In Non-Polar Compounds |
| Takes Place In | Metals & Non-Metals | Non-Metals Only |
Similarities between Ionic and Covalent Bond
The following are the similarities between the ionic and covalent bonds.
Ionic and Covalent Bond Characteristics
Both Ionic and covalent bonds complete their outermost shell with eight electrons. In other words, they follow a complete octet rule.
Same Bonding Techniques
Both have the same bonding method techniques. As the two atoms come close to each other, the nuclei of both atoms attract each other. Furthermore, the bond will have a high covalent character if the electrons’ attractions of the neighboring atoms and the nuclei of bonded atom are the same. When the attraction becomes more polarized, the bond considers having an ionic character.
Ion Formation by Electrostatic Force of Attraction
Ions are formed by the electrostatic force of attraction in both ionic and covalent bonds. In the case of ions, anions and cations are positive and negative charges formed by losing or gaining electrons. While in a covalent bond, electrons in the valence shell are shared directly between the atoms.
Provides Stability
Both bonds give stability to the bonded atoms than individually and in both electrons of the valence shell take part while making the bond.
Formation of Neutral Bonds
The molecules or compounds that are formed from the ionic and covalent bonds are neutral. This is because, in an ionic bond, two opposite charges cancel each other while in a covalent bond, neutral components shared the pair of electrons.
Exothermic Processes
Ionic and covalent, both are exothermic processes. When two elements make bonds (ionic or covalent) lower their potential energy. During this reaction, energy is released in the form of heat.
Sharing of Fixed Electron Quantity
Both ionic and covalent bonds share fixed quantities during bond formation. In an ionic bond, an excessive charge is found on the elements. A fixed amount of ions that join together to make the compound, depends on this excessive charge on the ions. In a covalent bond, elements shared the fixed amount of electrons that they needed to complete their valence shell.
Regular Pattern
Both show the regular pattern and structure in a solid or crystalline state.
Alters Shape with Temperature and Pressure
When both compounds face the right temperature and pressure, they change their shape physically.
Conclusion
Both the bonds, ionic and covalent, are critical to organic chemistry. Scientists use ionic properties of the respective compounds to form the desired products. Similarly, covalent bonding creates long chain of compounds, particularly the carbon compounds, giving more complex structures.

Amna being a Physicist wants to share her subject expertise and knowledge with people. Amna is a highly energetic and motivated lecturer as well.