All organisms use cellular processes like fermentation and cellular respiration to produce energy for body functioning. All these processes take place in different parts of the prokaryotic and eukaryotic cells. Fermentation is an important cellular activity that contributes to the breakdown of glucose to generate energy. It may produce different end products, leading to its categorization.
Here’s everything you need to know about the types of fermentation.
What is Fermentation?
The origin of fermentation is the Latin verb ‘fevere,’ which means ‘boil.’ The term “fermentation” refers to a broad category of metabolic processes in which the action of microorganisms results in energy production in the body. It may also industrially contribute to an improved food or drink in some way, be it in taste, shelf life, or health benefits. Ironically, fermentation may occur without the use of heat. The name comes from the appearance of boiling caused by the constant expulsion of gas bubbles to the surface during the beginning of wine fermentation.
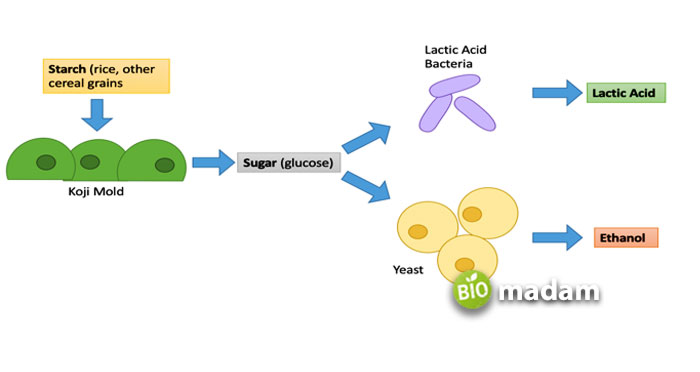
Fermentation in Different Fields
- Microbial Physiology: The term fermentation refers to the metabolic pathway of a carbon source in which ATP production by substrate-level phosphorylation (one of the three mechanisms of phosphorylation) occurs.
- Industrial Microbiology: It defines fermentation as any process in which microorganisms cultivation takes place on a large scale, even if the ultimate electron acceptor is not an organic substance (i.e., if the growth happens under aerobic conditions) Even in oxygen-rich environments, yeast cells (Saccharomyces cerevisiae) prefer fermentation over aerobic respiration.
- Food Microbiology: Food microbiology describes fermentation as any metabolic process in which microorganisms’ activity generates a favorable change in food and beverages, whether it’s boosting flavor, preserving foodstuff, or delivering health benefits.
Types of Fermentation
Fermentation is categorized into three types depending on the end product they generate.
Ethanol Fermentation
One glucose molecule yields two ethanol and two carbon dioxide molecules in ethanol fermentation. The following chemical equation illustrates the alcohol fermentation of glucose (C6H12O6):
C6H12O6 → 2C2H5OH + 2CO
One glucose molecule splits in half to create two pyruvate molecules before fermentation. This process is glycolysis. Starches or sugars contain pyruvate molecules, which yeasts convert to alcohol and carbon dioxide through glycolysis, the breakdown of glucose (C6H12O6). Beer and wine are both the results of alcohol or ethanol fermentation.
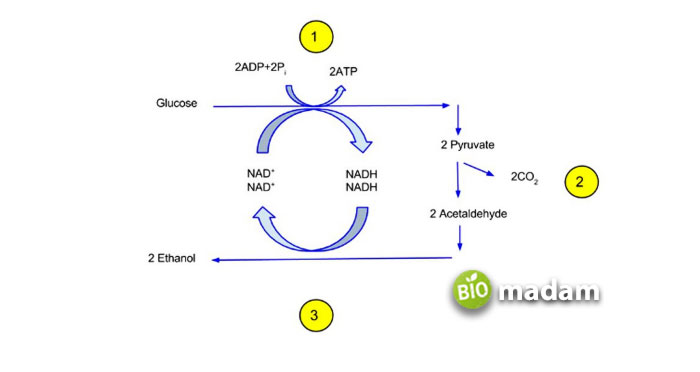
Lactic Acid Fermentation
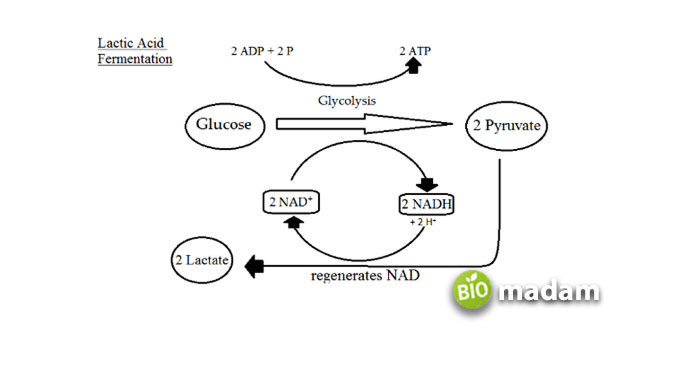
Lactic acid fermentation refers to the breakdown of glucose molecules to produce lactic acid. It is further classified into two types.
Homolactic Fermentation
It is the simplest form of fermentation that only produces lactic acid. During glycolysis, pyruvate undergoes a simple redox reaction to create lactonic acid. It stands out from other forms of respiration in that it does not generate waste gasses. In the end, one glucose molecule produces two molecules or compounds:
C6H12O6 → 2CH3CHOHCOOH
This condition develops when an animal’s muscles need energy quicker than the blood can deliver oxygen. Some yeast and bacteria exhibit this property. Yogurt acquires its characteristic sour flavor from a specific strain of bacteria that ferments the milk sugar lactose into the acid lactic acid. Most lactic acid bacteria ferment in homolactic fermentation.
Heterolactic Fermentation
Some lactate exhibits fermentation in a heterolactic microorganism, leading to a different metabolic product, such as ethanol and carbon dioxide (through the phosphoketolase route), acetate, or others.
C6H12O6 → CH3CHOHCOOH + C2H5OH + CO2
Fermentation breaks down lactose into glucose and galactose (two sugars with the same atomic formula), which are then used to produce dairy products like yogurt and cheese. A mixture of C12H22O11 and water has two molecules of C6H12O6.
Compared to lactic acid fermentation and other types of fermentation, like alcoholic fermentation, heterolactic fermentation occupies a middle ground. Extra steps to transform lactic acid into anything else are warranted for the following reasons:
- Because of the lactic acid’s acidity, biological processes are halted. Fermentation can be advantageous to the organism because it drives out competitors who are not used to the acidity, providing the food with a longer shelf life (part of the reason fermentation takes place in the first place). Still, after a certain point, the acidity starts to harm the organism that produces it.
- Le Chatelier’s principle states that a high concentration of lactic acid, the result of fermentation, causes the equilibrium to shift, slowing growth and reducing the rate at which fermentation may take place.
- It can rapidly change Lactic acid to ethanol, which is volatile and will easily escape, facilitating the reaction. Additionally, it produces CO2. However, it is considerably more volatile than ethanol and only mildly acidic.
Acetic Acid Fermentation
Fermentation of the starches and sugars in grains and fruit results in vinegar and condiments with a sour flavor. Apple cider vinegar, wine vinegar, and kombucha are just a few examples. Acetic acid fermentation takes place in two steps:
- Ethyl alcohol is first produced by fermenting sugar without oxygen using yeast.
- Second, acetobacter bacteria oxidize the ethyl alcohol into acetic acid. Alcohol oxidation by microbes results in an acid.
The process can further be divided into two kinds according to utilization.
- Primary: In this phase, microbes work quickly on raw ingredients like fruit, vegetables, or dairy. E.g., yeast or microorganisms turn glucose into alcohols and acids.
- Secondary: In this lengthier stage of fermentation, alcohol levels rise, yeasts and bacteria die, and their food source (carbohydrates) becomes limited. Secondary fermentation creates wine and beer.
The Bottom Line
Fermentation is a common process in different eukaryotic cells that contributes to energy production by breaking down sugars. Fermentation typically takes place through bacteria and yeast, further used in baking. Ethanol, lactic acid, and acetic acid fermentation are three types of fermentation depending on their end product. These processes could involve several steps leading to the breakdown of sugar molecules to liberate energy. They are used in food industries to enhance flavors and microbial research to understand the role of microorganisms in our ecosystems and communities.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.

