Recently updated on March 8th, 2023 at 07:47 am
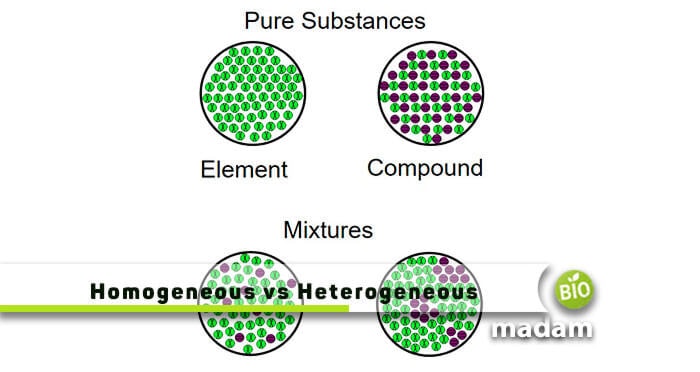
Here to find the difference between homogeneous and heterogeneous mixtures. When two or more substances (molecules or compounds) mix together without showing any chemical change, they are called mixtures. It generally has two major types based on the mixing of substances, such as homogeneous mixtures and heterogeneous mixtures.
You can say that in mixtures, there is a thorough mechanical blending without any interference with the composition of substances. The resulting component doesn’t lose its identity and continues to show its properties. Some common examples of mixtures are a mixture of salt and sugar, a mixture of water and lime juice, etc.
Before finding the difference between homogeneous and heterogeneous mixtures, we’ll quickly take a glance at their comparison chart. So, let’s begin!
Comparison Table
| Basis of Comparison | Homogeneous Mixture | Heterogeneous Mixture |
| Phases | Exists in only one phase | Can have two or more two phases |
| Composition | Shows uniform composition | Doesn’t show the uniform composition |
| Physical Separation | Cannot be physically separated | Can be separated physically |
| Other Names | Also called “Solutions” | Also called “Colloids/Suspensions |
| Size of Particles | Usually, particles are smaller in size | Generally bigger particles than a homogeneous mixture |
| Examples | Natural gas, air, juices, etc. | Pizza, chocolate chip cookies, chicken soup, etc. |
Explain Homogeneous Mixture
The type of mixture where substances are uniformly mixed and distributed everywhere is called a homogeneous mixture. The word homo means “same,” hence, these mixtures have a uniform consistency. There is a single phase of components inside a homogeneous mixture. Some key points relating to it are as follow:
- Such mixtures are also called solutions.
- They have a uniform composition.
- Nobody can examine a homogeneous mixture by just looking at it.
- For instance, vinegar, air, most alloys, etc.
Explain Heterogeneous Mixture
Another type of mixture where, after thoroughly mixing the substances, all particles are observed under a microscope is called a heterogeneous mixture. There is more than one phase in a heterogeneous mixture, so it’s easy to examine its substances through a microscope. A few key points relating to this mixture are as follow:
- Such mixtures are also called suspensions.
- They do not have a uniform composition.
- One can easily examine a heterogeneous mixture by just looking at it.
- For instance, chicken soup, sand, water, etc.

Discover the Differences Between Homogeneous & Heterogeneous Mixtures
We already have taken a general idea about both types of mixtures above. But to know more about them, we have listed some key differences:
Literal Meaning
Homogeneous Mixture
Homogeneous is a Greek word that is composed of two words. “Homos” means “same/similar” and “genos” means “kind.”
Heterogeneous Mixture
Heterogeneous is another Greek word, composed of two words. “Hetero” means “different” and “genos” means “kind.”
Definition
Homogeneous Mixture
The mixing of two or more substances that results in uniform properties is called a homogeneous mixture.
Heterogeneous Mixture
The mixing of two or more two substances that do not result in uniform properties is called a heterogeneous mixture. One can see the different particles added in a heterogeneous mixture.
Number of Phases
Homogeneous Mixture
This mixture shows only a single phase, which means the homogeneous mixture has a uniform composition.
Heterogeneous Mixture
Heterogeneous mixtures appear with two or more phases, meaning there is no consistency in their composition. It can be either in a combination of solids and liquids, solids and gases, liquids and gases, or maybe all three at a time.

Ability to Separate
Homogeneous Mixture
Because of its uniformity, this mixture can never be physically separated.
Heterogeneous Mixture
One can easily separate a heterogeneous mixture physically as the particles inside are all separated.
Solutions & Suspensions
Homogeneous Mixture
These mixtures are also called solutions; however, suspensions can sometimes be homogeneous too.
Heterogeneous Mixture
These mixtures are also known as suspensions or colloids, unlike solutions.

Utilization of Microscope
Homogeneous Mixture
The components of a homogeneous mixture are not seen with the naked eye, so using a compound or electron microscope is a must.
Heterogeneous Mixture
The components in a heterogeneous mixture can easily be seen with the naked eye, but for better results, you can utilize a student microscope. A compound microscope must be enough to observe colloidal particles.
Examples
Homogeneous Mixture
Some examples of homogeneous mixtures are alloys, seawater, brass, vinegar, air, blood, natural gas, etc.
Heterogeneous Mixture
Salt and pepper together make a heterogeneous mixture. Other examples include soda, concrete, sand and sugar, muddy water, chocolate chip cookies, all kinds of suspensions, etc.
Conclusion
We tried to cover all major points by presenting the differences between homogeneous and heterogeneous mixtures above. Both types, homogeneous and heterogeneous, are the essentials of an environment. You can simply analyze the nature of a mixture through its sample size. If the sample shows multiple phases, it is definitely a heterogeneous mixture. In an otherwise case, the mixture is entirely homogeneous.

Hello, I would like to introduce myself to you! I am Chelsea Rogers, an experienced blog writer for science articles, holding an MPhil degree. My enthusiasm to grab the best knowledge, let it relate to botany, zoology, or any other science branch. Read my articles & let me wait for your words s in the comment section.