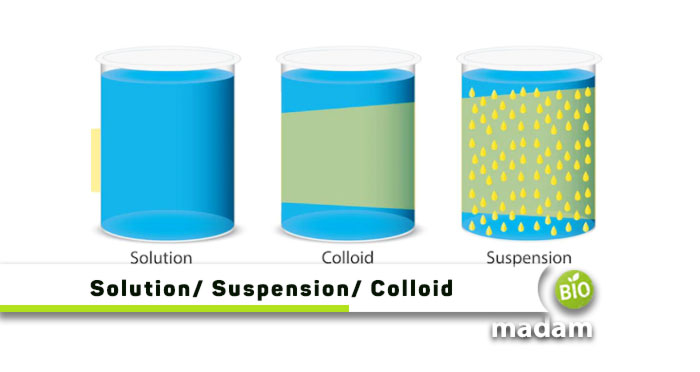
Solutions, suspensions, colloids, mixtures, liquids, and aqueous are commonly heard terms in physics and chemistry. Understanding the unique properties of all these different substances helps you understand their role in chemical reactions. It gives information about their physical and chemical properties. It also lets researchers and students study the relationship between different substances. Keep reading to learn all the differences between solution, suspension, and colloids.
Comparison Table
| Characteristics | Solution | Suspension | Colloids |
| Definition | Dissolved particles | Dispersed particles | Floating particles |
| Solution Type | Homogeneous | Heterogeneous | Heterogeneous |
| Particle Type | Small | Large | Moderate |
| Particle Size | < 1nm | > 100nm | 1nm – 100nm |
| Tyndall Effect | Absent | Present | Present |
| Separation by Filtration | No | Yes | Yes |
| Stability | Yes | No | Yes |
| Examples | Salt and sugar solution | Sand in water | Blood and milk |
What are the Solutions?
A solution is any homogeneous mixture of solutes and solvents. The term “solvent” refers to the substance in which a solute can dissolve to produce a homogeneous mixture. A solution results when a solute dissolves in a solvent. “Solute” refers to a substance that dissolves in a solvent. Take into account that the solvent is the component in the higher quantity. There is a large variety of possible solutions. A solute, for instance, can come in the form of a gas, a liquid, or even a solid. A solvent also has three possible states: a gas, a liquid, or a solid.

Properties of Solutions
- Substances in a solution remain combined evenly throughout.
- It has particles with a diameter of less than 1 nm, making it too small to see with the naked eye.
- A particle can go undetected if a light beam can pass through it. It is because the particle does not alter the light’s trajectory.
- Insoluble substances do not settle at the bottom, and the solution does not change over time.
- Filtration doesn’t separate ingredients present in solutions.
Types of Solutions
- Based on the Nature of Solvent: Solutions can be aqueous or non-aqueous. Aqueous solutions contain water; e.g., sugar in water is an aqueous solution. Non-Aqueous solutions don’t contain water like ether and benzene.
- Based on Solute Added: Unsaturated solutions dissolve more solute at specific temperatures than saturated. It is possible to combine the solute with the solvent. When we cannot add more solute to a solution, we say it’s saturated. At a specific temperature, the solvent cannot dissolve more solute anymore. Supersaturated solutions have excess solute. Increasing temperature or pressure dissolves this solute.
- By the Amount of Solvent: The solution is concentrated when there is a high amount of solute in a relatively small volume. Examples include brine, orange, juice, and dark tea. Alternatively, dilute solutions have a small amount of solute in a high solvent volume. Salt solution and light tea are examples of dilute solutions.
Examples of Solutions
- Sugar water
- Rubbing alcohol
- Mouth wash
- Alloys
- Air
What are Suspensions?
Solutions and suspensions differ from each other due to the size and visibility of particles. In a suspension, the solid particles do not completely dissolve in the liquid solution, making it a heterogeneous mixture. Despite repeated attempts to mix them into a solution, materials in a suspension may be insoluble.
Most suspensions are non-homogeneous systems in which solids disperse in liquids. Suspensions consist of particles that are large enough for gravity to extract them from the dispersing medium. The dispersion medium can be easily filtered to remove these particles.

Properties of Suspensions
- The solute particles in a suspension are relatively large. Particles in a suspension are typically more than 100 times larger than those in a solution.
- One can see the individual particles that make up a suspension.
- Filter paper does let the particles of a suspension through to the other side. Filtration can separate a suspension in this way.
- As a result of the large particle size in a suspension, it will scatter any light that enters it.
- There needs to be more stability in the suspension. Over time, suspended particle suspension will begin to sink to the bottom.
Types of Suspension
Suspensions can be of different types, including water and non-water (non-aqueous) suspensions. They are typically used for research purposes.
Water-based Suspensions: As the name indicates, these suspensions comprise water as the base. They contain a solute that does not dissolve completely in water. Sand suspended in water or muddy water are examples of water-based suspensions.
Non-aqueous Suspensions: Non-aqueous suspensions do not contain water as the solvent. Instead, they use a non-aqueous solvent that may be polar or nonpolar. Glycols, alcohol, glycerol, and esters are mostly used polar solvents in non-aqueous suspensions. On the other hand, nonpolar suspensions may contain hydrocarbons.
Suspensions are also categorized based on their applications in the pharma industry. The classification of suspensions according to their route of administration includes oral, otic, ophthalmic, topical, and nasal suspensions.
Examples of Suspensions
- Chalk in water
- Mashup paints
- Milk of magnesia
- Lime and water
- Thousand island dressing
What are Colloids?
Colloids are mixtures in which one substance is broken into tiny particles dispersed throughout another. These tiny droplets are known as colloids. We can also characterize colloids as solutions with solute particles between 1 nm and 1 m in size. The composition of colloids varies widely. People often think of water as a colloid, but unless it contains other minerals, it is a pure substance.
Properties of Colloid
- Colloids are not homogeneous solutions.
- They are even smaller than human hair as colloidal particles are incredibly minuscule.
- They exhibit Tyndall’s influence, i.e., it disperses the light and reveals how it traveled through it.
- Colloids don’t calm down even when given plenty of time. That stability is a hallmark of colloidal solutions.
- Filtration cannot separate colloidal particles from the solvent.
- Centrifugation can be used to separate them.
- The motion of subatomic particles, such as those in colloidal suspensions, is Brownian.
Types of Colloids
Sol: Sols are solutions in which microscopic solid particles dissolve in a liquid. The particles are the least visible among all forms of colloids.
Emulsion: Emulsions are a colloidal mixture consisting of two or more liquids, each containing a dispersion of the other liquids.
Foam: Foam is a mix of microscopic bubbles of gas suspended in a more solid or liquid medium. The gas divides the liquid into a surface with tiny bubbles appearing on the top.
Aerosol: Little liquid or stable droplets suspended in a gaseous medium make up an aerosol. Aerosols may result from physical or chemical reactions in the environment. Synthetically produced aerosols are also used to deliver medicines like asthma inhalers.
Examples of Colloids
- Toothpaste
- Silver iodide sol
- Blood and plasma
- Inhalers
- Smog
Difference between Solution, Suspension, and Colloid
Definition
Solution
A solution is a combination of solute and solvents in which the solute particles completely dissolve in the solvent.
Suspension
Suspensions refer to mixtures that do not look homogenized due to the inability of the solute particles to dissolve in the solvent medium.
Colloid
Colloids are similar to suspensions and contain ultramicroscopic particles floating in another system.
Type of Solution
Solution
A solution mixture is homogeneous.
Suspension
The mix of suspensions is heterogeneous, but sometimes, suspensions can be homogeneous too.
Colloids
On the other hand, colloids are heterogeneous mixtures.
Size of Particles
Solution
Particles are extremely diminutive in size, smaller than 1 nm.
Suspension
In contrast, the particles are comparatively larger, more than 100 nm.
Colloid
Particles in colloids are of moderate size, not too small or too big; ranging between 1 and 100 nm.
Tyndall Effect
Solution
Solutions do not exhibit the Tyndall effect as the particles do not disperse light.
Suspension
Suspension particles diffuse or scatter the light, giving the Tyndall effect.
Colloid
Colloidal particles scatter light, leading to the exhibition of the Tyndall effect.
Separation through Filtration
Solution
The solute particles are evenly distributed and dissolved in the mixture, making it difficult to separate them through filtration.
Suspension
The particles in suspensions may be separated through filtration.
Colloid
In addition, it is possible to separate colloidal particles through filtration.
Stability of Particles
Solution
The particles in solutions remain equally dissolved in the system and do not split up.
Suspensions
Suspensions are not as stable as solutions or colloids.
Colloids
The particles in colloids remain dispersed throughout and exhibit movement.
Examples
Solution
Sugar in water and salt in water are examples of solutions.
Suspension
A mixture of sand and water and a mix of chalk and water are suspensions.
Colloid
Examples of colloids include milk and blood.
The Bottom Line
Solutions, colloids, and suspensions are important terms in different branches of chemistry. They refer to different types of homogeneous and heterogeneous mixtures with unique properties. The main difference between solutions, suspensions, and colloids is the difference in their particle size. The particles in solutions dissolve properly in the solvent, while suspensions and colloids contain dispersed particles.
FAQs
What is the difference between a colloid and a suspension?
While suspensions and colloids both have suspended particles, the particles in the colloid stay suspended. On the other hand, suspension particles may settle down at the bottom when standing for long.
What are 3 examples of colloids?
Common examples of colloids include smog, pigmented plastics, Au sol, and sprays. They have small particles ranging between 1 nanometer and 10 micrometers that stay suspended without an external force.
Is fog a suspension?
Some classify fog as a suspension that contains water vapors in the air. However, it is typically considered a colloid with water vapors as the solute and air as the solvent. The water vapors are distributed throughout the air.
Can you call colloid a solution?
Though colloidal particles do not settle down like suspensions, they are not solutions. Homogenous solutions must not have any undissolved particles, unlike colloids.
Is every mixture a solution?
Generally, mixtures are referred to as heterogeneous solutions. Thus, all mixtures are not solutions. The solute in a solution must dissolve thoroughly in the solvent. However, mixtures or heterogeneous solutions do not have completely dissolved solutes.

Meet me; I am Paulina Zaniewska, who’s more hooked on providing the best health blog. I’ve always been so determined to compete as a nutritionist, and here I am, done with a Master’s in food technology. My brilliant performance throughout encouraged me to help people.