Recently updated on March 7th, 2024 at 01:02 pm
Every DNA strand has a backbone made up of a sugar-phosphate chain. A nitrogenous base, composed of carbon and nitrogen rings, is attached to each one of these sugars. The number of rings of the attached base determines whether the base is a purine (two rings) or a pyrimidine (one ring).
These are the most important building blocks of DNA and RNA. They are the nitrogenous bases that make up the nucleic acids. Today, we are here to discuss the clear differences between the two bases despite the that they both work together to recognize a human’s hereditary characteristics.
Before getting into the details of the differences between purine and pyrimidine, let’s start with their nomenclature and a brief comparison table.
Comparison Table
| Basis of Comparison | Purines | Pyrimidines |
|---|---|---|
| Chemical Formula | C5H4N4 | C4H4N2 |
| Size | Larger | Comparatively Smaller |
| No. of Rings | Two | One |
| Solubility | Soluble in Water | Insoluble in Water |
| Molecular Mass | 120.115 g/mol | 80.08 g/mol |
| Point of Biosynthesis | In Liver | In Several Body Tissues |
| Catabolism Products | Uric Acid | Ammonia, CO2, NH3 |
Nomenclature
Purines
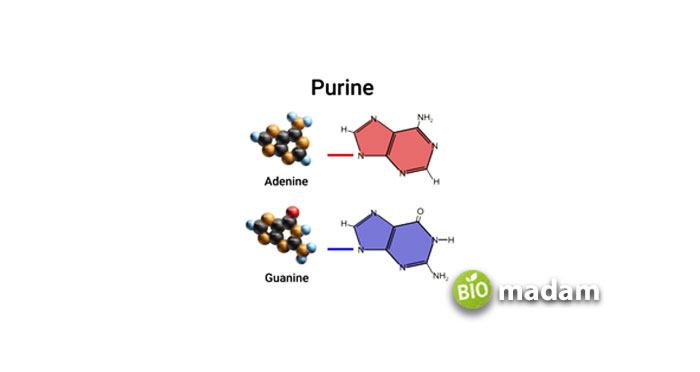
These are made up of a six-membered and a five-membered nitrogen-containing ring, which is fused. It consists of the following:
- Adenine—6-amino purine
- Guanine—2-amino-6-oxy purine
- Hypoxanthine—6-oxy purine
- Xanthine—2, 6-deoxy purine
Adenine and Guanine are found both in DNA and RNA. As Hypoxanthine and Xanthine are synthesized, they are not included in nucleic acids.
Pyrimidines
They have only a six-membered nitrogen-containing ring that is responsible for making double and triple bonds with purines. These areas follow:
- Uracil—2,4dioxy pyrimidine
- Thymine—2, 4-dioxy-5-methyl pyrimidine
- Cytosine—2-oxy-4-amino pyrimidine
- Orotic acid—2, 4-dioxy-6-carboxy pyrimidine
Cytosine is present in both DNA and RNA. Uracil is found only in RNA, and sometimes tRNA contains some Thymine along with Uracil. Thymine is normally found in DNA.
What is Purine
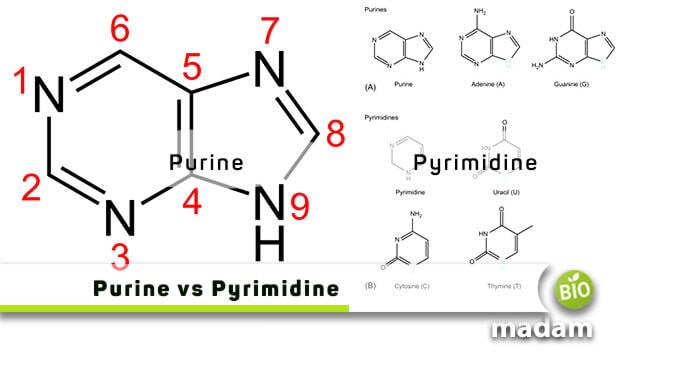
As mentioned earlier, purines are two-ringed structures where a pyrimidine ring fuses with an imidazole ring. It has a total of nine atoms, of which four are nitrogen and five are carbon atoms. We can differentiate several purine rings through tests as they differ by the functional group or atom attached to the corresponding ring.
Furthermore, these heterocyclic compounds are the most abundant ones, particularly in fish, meat, grains, peas, beans, etc. More examples of purines include xanthine, uric acid, caffeine, theobromine, and hypoxanthine. These molecules interlink with pyrimidines and are a significant part of RNA & DNA cells. Besides, purines are responsible for enzyme regulation, energy storage, and cell signaling.
What is Pyrimidine
These molecules, on the other hand, are composed of only a single ring. Pyrimidine is more of an organic molecule with four carbon atoms and only two nitrogen atoms. Biologists can easily prepare pyrimidine rings through organic materials and special techniques in the lab, for example, the Bigineli reaction.
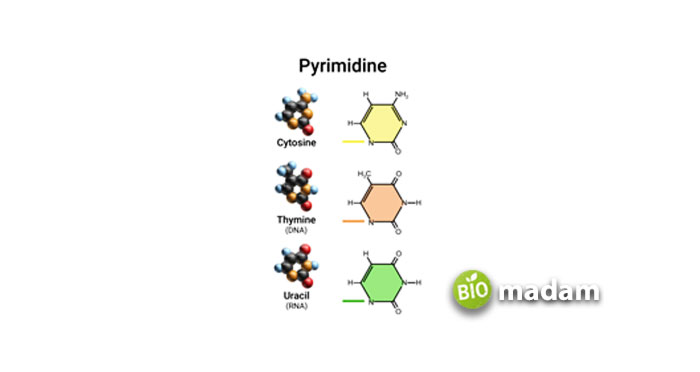
The atoms attached to the pyrimidine ring make it unique from others, which are
- Thymine
- Cytosine
- Uracil
- Uric acid
- Thiamine (Vitamin B1)
- Barbiturates, etc.
These organic molecules have a similar function as purines, so they are present in the DNA and RNA. Moreover, pyrimidines help store energy in the cell and also regulate enzymes.
Relationship between Purine and Pyrimidine
The purines form a hydrogen bond on one strand of DNA with the corresponding pyrimidine available on the opposite strand and vice versa to hold them together. It is called base pairing. It is the most important function of purines and pyrimidines within the DNA molecules. This hydrogen bonding is not as strong as a covalent bond. Therefore, this base pairing easily separates to allow DNA replication and transcription.
One strand of DNA is always an exact complement of the other as far as purines and pyrimidines go. The reason for this is, that purines always bind with pyrimidines, and this is called complementary pairing. Within a DNA molecule, the ratio of these two will always be constant. This phenomenon is called Chargaff’s Rule, named after Irwin Chargaff, who noticed this for the first time. It always remains constant as the atoms of purines pairing with those of pyrimidines are fixed.
- Adenine pairs with Thymine with two hydrogen bonds.
- Guanine pairs with Cytosine with three hydrogen bonds.
There is a way to remember this pairing. The letters made up of straight lines (A and T) are paired together, whereas the letters made up of curves (G and C) go together.
In a DNA molecule, the number of Adenines is always equal to the number of Thymine. The same is the case with Guanine and Cytosine. Therefore, if the percentage of one nitrogen base is known within a DNA molecule, the percentages of each of the other three can be figured out very easily. The complementary pair will have the same percentage, and the other two bases will each be the sum of the first pair subtracted from 100% and divided by two.
Purine vs Pyrimidine – Major Differences
Structural Difference
The most important difference between the two is in their structures.
Purines
As you can see in the figures below, these have two-ringed structures consisting of a nine-membered molecule with four nitrogen atoms.
Pyrimidines
On the contrary, pyrimidines have only one single ring with just six members and two nitrogen atoms.
Chemical Composition
Purines
We can even get an idea of purine’s composition through its structural difference from pyrimidines. They are composed of a pyrimidine ring, further attached to an imidazole ring. Therefore, a purine has two carbon-nitrogen rings.
Pyrimidines
In contrast, a pyrimidine ring is a single-structured ring with only two nitrogen atoms and four carbon atoms.
Nucleobases
Purines
They will always have the following four bases: guanine, adenine, xanthine, and hypoxanthine.
Pyrimidines
In contrast, pyrimidines have four bases, including thymine, cytosine, orotic acid, and uracil.
Catabolism Products
Purines
The catalysis of purines results in uric acid formation.
Pyrimidines
On the opposite, we can acquire beta-amino acid, ammonia, and carbon dioxide through pyrimidine catabolism.
The Intensity of Melting/Boiling Points
Purines
Due to being larger, it’s pretty difficult to melt or boil the compound. Hence, it ends up at higher melting and boiling points.
Pyrimidines
On the other hand, pyrimidines have comparatively lower melting and boiling points.
Conclusion
Despite differing characteristics, purines and pyrimidines always work together in DNA and RNA by making double and triple bonds. These are the essential nitrogenous bases in the human body, and we cannot healthily survive without them.

Hello, I would like to introduce myself to you! I am Chelsea Rogers, an experienced blog writer for science articles, holding an MPhil degree. My enthusiasm to grab the best knowledge, let it relate to botany, zoology, or any other science branch. Read my articles & let me wait for your words s in the comment section.

Very nice