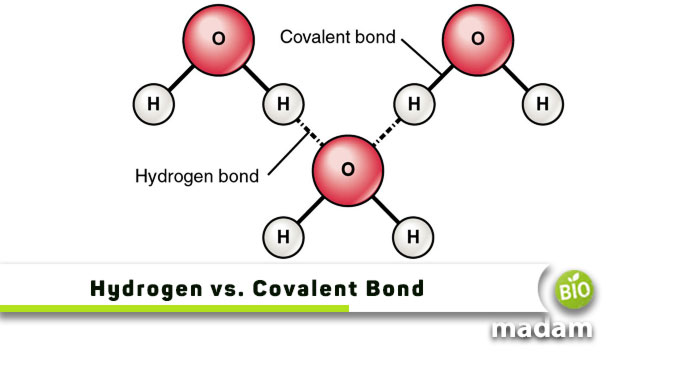
Chemical bonds are an important part of chemistry that helps us understand the composition of substances around us. Besides ionic and metallic, covalent bonds are also critical to studying atoms. Similarly, hydrogen bonds play a significant role in making up molecules and compounds. Although these bonds are weaker than covalent bonds, they still are integral to the structure of different objects around us. Did you know these bonds also contribute to structures within prokaryotic and eukaryotic cells? They are everywhere!
This article tells you everything about hydrogen vs. covalent bonds for better understanding.
What are Hydrogen Bonds?
Hydrogen bonds are weak bonds between two atoms within or among different molecules. They result from the electrostatic forces between a hydrogen atom (covalently) bonded to an electronegative atom. Thus, the hydrogen atom obtains a highly positive charge.
Hydrogen bonds typically form through the sharing of electrons. Compounds such as hydrogen fluoride and ammonia are formed when hydrogen is covalently attached to fluorine and nitrogen, respectively. Here, the sharing of electrons is uneven. Each electronegative atom obtains a negative charge with at least one active loan pair. The unshared electron pair in N, F, or O gives it a negative charge.
Hydrogen bond formation requires a hydrogen acceptor and donor. The electronegative atom acts as the donor, while the neighboring molecule with a lone pair is the acceptor. It is one of the major differences between hydrogen and covalent bonds.
Water has extensive hydrogen bonding in its structure, which is why it stays liquid over a wide range of temperatures. Its ability to form hydrogen bonds with numerous ionic compounds makes it a good solvent for most solutes.
Types of Hydrogen Bonds
Intramolecular Hydrogen Bonds
Intramolecular hydrogen bonds refer to bonds among the atoms or functional groups of one molecule. Like hydrogen bonding with other molecules, bonds within a molecule also require a donor and acceptor. For example, hydrogen bonds in water molecules.
Intermolecular Hydrogen Bonds
Hydrogen bonds among different molecules are called intermolecular hydrogen bonds. They may occur between the molecules of the same element or different. For example, water molecules may have hydrogen bonds with other water molecules. At the same time, they can have hydrogen bonding with other molecules like NH3.
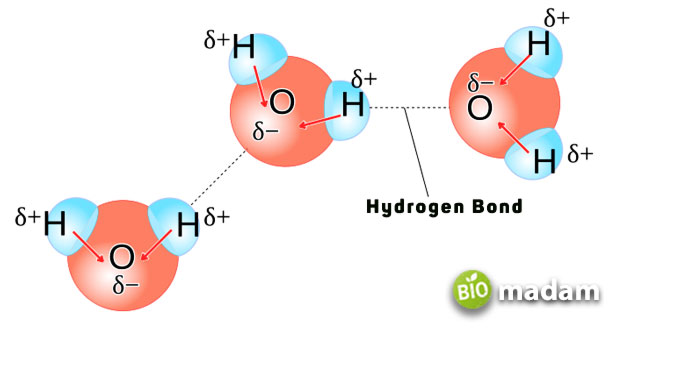
Hydrogen Bonding Example
Alcohol is one of the simplest yet most descriptive examples of hydrogen bonding. They form as a result of the –OH group bonding with other atoms. Hydrogen bonds due to -N-H or -O-H groups increase the boiling point.
While ethanol does not exhibit strong hydrogen bonding like water due to limited alcohol molecules, it elevates the boiling point to 78°C.
Before we jump to the differences between hydrogen and covalent bonds, here’s everything you need to know about covalent bonds.
What are Covalent Bonds?
Covalent bonds result from electron sharing among two atoms. These bonds do not require an acceptor or donor, as none of the atoms give away their electrons.
These bonds are strongly formed by sharing electrons in their outermost shell with another atom.
Nonmetals widely form covalent bonds with other nonmetals to get stable; they may have more than one covalent bond depending on the number of valence electrons. Also, most covalent bonds exhibit unequal sharing of electrons among the molecules. While the phenomenon is explained as “electron sharing,” typically, only one atom offers the electrons.
The following types of covalent bonds result from electron sharing between atoms:
Single Bond: Single bonds are formed by sharing one electron pair between two atoms. Single covalent bonds are weaker than double and triple but they are the most stable. Thus, they exhibit lower reactivity.
Double Bond: The sharing of two electrons between atoms results in double covalent bond formation. They are somewhere between single and triple bonds in terms of stability, reactivity, and strength.
Triple Bond: Triple bonds result from the sharing of three electrons between two atoms. They are the least stable yet the strongest bonds among all three.
Most atoms share one to three electrons at most, giving rise to these three types of covalent bonds. However, you may see an uncommon exhibition of more than three bonds.
Types of Covalent Bonds
Polar Covalent Bonds
Polar covalent bonds arise from the unequal sharing of electrons between two molecules. This typically occurs when one atom has a lower electronegativity than the sharing atom. So, the atom with the higher number of electrons will pull the electron towards itself, leading to higher electronegativity, leading to unequal sharing. Polar covalent bonds have an electrostatic potential due to a slight negative and slight positive charge on the other atom. This electrostatic potential makes the overall molecule polar.
Non-Polar Covalent Bond
When an atom shares electrons with another atom with the same electron affinity, it forms a nonpolar covalent bond; none pulls the electron with a higher force when the atoms have the same affinity. Thus, it forms nonpolar covalent bonds. Nonpolar covalent bonds are common in gas molecules.
Covalent Bond Example
Carbon is an interesting example of covalent bond formation, having four covalent bonds. Carbon has four valence electrons, and losing or gaining 4 electrons for ionic bonding is nearly impossible. So, the atoms complete their octet by sharing electrons.
Hydrogen Bond Vs. Covalent Bond
Now that you know all about hydrogen and covalent bonds in detail, let’s tell you the main differences between hydrogen and covalent bonds.
Definition
Hydrogen Bond
Hydrogen bonds result from the electrostatic attraction between hydrogen and an electronegative atom.
Covalent Bond
In contrast, the sharing of electrons between atoms leads to the formation of covalent bonds.
Representation
Hydrogen Bond
Hydrogen bonds are represented by a dotted line between the hydrogen and electronegative atom, i.e., H···Y.
Covalent Bond
In chemistry, you represent covalent bonds with a single, double, or triple line, according to the nature of the bond, i.e. -, =, and ≡.
Strength
Hydrogen Bond
When observing the hydrogen vs. covalent bond strength, the first one has typically weaker bonds than the covalent as the sharing of electrons is uneven.
Covalent Bond
Whereas, on the contrary, sharing electrons between atoms results in higher strength of covalent bonds.
Bond Type
Hydrogen Bond
Hydrogen bonds are secondary bonds formed after a covalent bond.
Covalent Bond
On the contrary, covalent bonds are the primary bonds that are much stronger than any other bond. All this chemical bonding is due to the complete sharing of electrons between the two covalently bonded atoms.
Classification
Hydrogen Bond
Hydrogen bonds are classified into intramolecular and intermolecular bonds.
Covalent Bond
Meanwhile, covalent bonds can be of different types. These are single, double, triple, polar, and nonpolar covalent bonds.
Conditions
Hydrogen Bond
Hydrogen bonds form only between hydrogen and an electronegative atom. It means, one atom is highly electronegative, whereas the other would always be a proton (hydrogen ion).
Covalent Bond
At the same time, covalent bonds may form between any atoms having shareable electrons.
Number of Atoms
Hydrogen Bond
Hydrogen bonds may involve more than one atom or molecule.
Covalent Bond
Conversely speaking, covalent bonds form between two atoms that have the tendency to share electrons.
Influence on Properties
Hydrogen Bond
Hydrogen bonds impact the physical properties of the bonding components.
Covalent Bond
In contrast, a covalent bond alters the chemical properties of the bonding elements.
Bond Strength
Hydrogen Bond
Hydrogen bonds are weaker bonds and have a lower bond strength of 5 to 50 kJ/mol.
Covalent Bond
Contrarily, covalent bonds are stronger, exhibiting a higher bond strength of 100 to 110 kJ/mol.
Example
Hydrogen Bond
The water compound is the most common example of a hydrogen bond (H···O). Other examples of hydrogen bonding include HF, NH3, and alcohol.
Covalent Bond
Similarly, there are numerous examples of covalent bonds. For example, all carbon atoms are covalently bonded to each other. Other examples include H₂, O₂, N₂, and methane (CH₄).
Hydrogen and Covalent Bonds in DNA

DNA is a double-helix structure packaged inside a chromosome, having complementary DNA bases on each helix. Covalent bonds strongly connect the bases, sugars, and phosphate groups. On the other hand, hydrogen bonds join the two strands of the double helix of the DNA. The hydrogen atoms attached to molecules on one DNA strand form hydrogen bonds with the oxygen or nitrogen on the other strand.
The Bottom Line
All objects around us comprise atoms and molecules held together by chemical bonds, including ionic, covalent, hydrogen, and metallic bonds. Hydrogen bonds are weaker bonds formed by hydrogen atoms, while covalent bonds are strong bonds resulting from electron sharing. Moreover, hydrogen bonds do not include electron sharing; it is the most significant difference between hydrogen and covalent bonds. Water is the most common example of hydrogen bonding, while hydrogen, carbon, and oxygen molecules exhibit covalent bonding.
FAQs
Why is a hydrogen bond weaker than a covalent bond?
Hydrogen bonds result from electrostatic forces between two atoms, whereas covalent bonds require sharing of electrons between the atoms. Thus, hydrogen bonds are not as strong as covalent bonds.
What is the difference between hydrogen bonds and covalent bonds in DNA?
Covalent bonds in DNA connect the sugars, bases, and phosphate groups together, while hydrogen bonds join the base pairs on one strand of the helix to the other.
Which type of bonding is strongest?
As ionic bonds involve the complete transfer of electrons from one atom to another, it is the strongest type of bond among others.
How many times a covalent bond is stronger than an H-bond?
Covalent bonds are around 20 times stronger than hydrogen bonds due to the sharing of electrons between the atoms.

Anna has completed her degree in Pharmacy from the University of Hawaii. She is serving as a research assistant in a pharmaceutical company. She had a great interest in writing blogs, traveling to different parts of the US, and trying delicious recipes in her spare time.