Chromatography is among the best analytical techniques to separate a single mixture into its components. Different chromatographic techniques are utilized, but they all work on the principle to separate, identify, and purify the mixture components for qualitative and quantitative analysis.
Liquid chromatography is one of the primary types used to separate a liquid sample into its components, just like an ink is spread on the paper and separated. It further has different sub-types based on the analysis of materials; one is called affinity chromatography.
This article helps you briefly understand affinity chromatography and its significant disadvantages. So, let us first check what affinity chromatography is.
What is Affinity Chromatography?
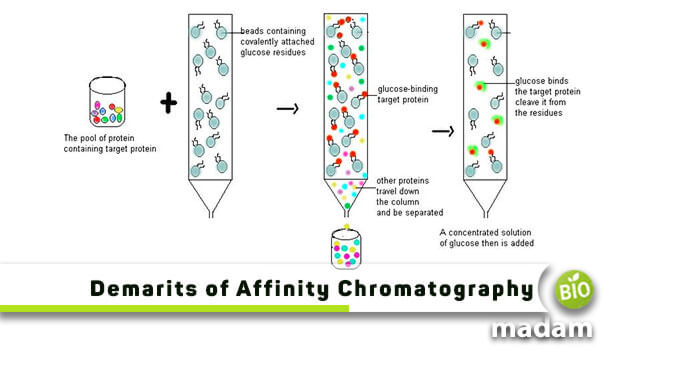
Cuatrecasas, along with Anfinsen and Wilcheck, in 1968 first described affinity chromatography. They explained it as a procedure involving reversible adsorption of protein to a ligand fixed on a matrix. This interaction is exclusively bio-specific and contains pairs such as receptor-ligand, enzyme-inhibitor, antibody-antigen, nucleic acid binding protein, and hormone-receptor.
Besides having prominent advantages like high sensitivity and selectivity, maintenance of quality and purity, and reproducibility, affinity chromatography disadvantages are also . Researchers who plan to practically involve this type of liquid chromatography in their schedules should consider these limitations. Let us try to understand them one by one in detail:
Disadvantages of Affinity Chromatography
If you narrow it down, you can come across numerous disadvantages of affinity chromatography. However, we have enlisted the major ones with their practical solutions below:
Demands Skills as a Necessity
Affinity chromatography is a highly specific technique that depends on interactions between target molecules and an immobilized ligand for separation and purification. This specificity demands choosing the right ligand, correctly surrounding the chromatography column, and optimizing its conditions. Besides, it stresses good analytical as well as hands-on expertise for its successful execution, including buffer preparation, sample preparation and application, elution, maintenance of flow rates, and analysis of the results. Overall, all these steps require one to be good at calculations, organized concerning time management, and a certain level of expertise.
Good technical skills are also required for proper column packing for affinity chromatography. So, this becomes a tedious and time-consuming technique, especially in the case of larger samples. Moreover, constant attention must be devoted while experimenting, such as continuously adding the solvent to keep the adsorbent covered and frequently switching vessels for sample collection. These required skills become a limitation and can lead to suboptimal results.
Sample Volume Limitations
The sample volumes need to be judiciously adjusted while performing affinity chromatography. For example, sometimes, a researcher has a sample containing lower component concentrations to be separated. In such cases, it is necessary to cut down the sample volume via concentration techniques to make it more efficient.
On the other hand, if one has a sample with highly concentrated amounts of the substance or mixtures to be separated, it is logically a good practice to dilute the samples at concentrations at which the experimenter gets an excellent and efficient separation of all required.
Non-Specific Binding
Although affinity chromatography is known for its specificity, non-specific binding can still occur, resulting in impurities in the eluted fractions. It gets challenging to take control of this immediate binding, especially when handling complex biomolecule samples.
There are ways to overcome this issue, such as optimizing buffer conditions, changing pH strengths, and minimizing interactions between the target molecule and unwanted contaminants. Moreover, deep cleaning of the chromatography columns before using it can also help as this step removes non-specifically bound impurities.
Purification Challenge
Therapeutic proteins and vaccines are most commonly produced utilizing recombinant technologies. For this reason, they often lack a common structural element required to capture them via affinity chromatography. It poses a unique challenge for the purification of such proteins via affinity chromatography.
One way to overcome this problem is to specifically structure and make ligands for such purposes. However, for many such targets, it is challenging (in some cases impossible) to prepare viable affinity candidate ligands. Moreover, in some cases, artificial ligands can be utilized. Still, this is a significant disadvantage associated with this technique.
Protein Loss
Proteins are usually taken as samples, so their loss is considered a major disadvantage associated with affinity chromatography. It happens in such a way that, in some cases, the affinity column or wells of the plate become clogged due to cell debris that might be present in a sample.
In such scenarios, the protein to be purified remains attached to the cellular debris instead of their ligands. Therefore, a considerable amount of sample is lost during clarification/elution steps, leading to major reductions in final yield.
Researchers can overcome this problem by carefully optimizing the buffer conditions and ligand density to minimize the protein denaturation risks. Moreover, they can work on concentrating eluted fractions through processes like ultrafiltration, which will ultimately increase the yield of purified biomolecules.
Costly Ligands
Affinity chromatography relies on the specific interaction with a ligand bound to the resin to make the chromatographic column. These ligands and their production are costly due to ligand synthesis, using specialized equipment and suitable column materials. All of these can limit the accessibility of specific affinity chromatography.
For example, in most cases, the ligands are antibodies against a specific protein. These types of antibodies must be manufactured before starting the protein purification experiment.
Researchers can overcome this issue with collaborative efforts. They should shift to cost-effective procedures, such as using shared equipment from the laboratories or using pre-packed columns to reduce the overall cost burden.
Ligand Leakage
Ligand leakage is a process in which the ligand attached to resin is detached from it, decreasing the efficiency of the resin and ultimately making it useless. It is generally observed in the affinity matrices, and its extent depends on various factors. For example, one influencing factor for ligand leakage is its coupling efficiency with the resin. The lower this efficiency, the higher would be the leakage.
Other factors affecting ligand leakage can be special conditions of the process used to carry out affinity chromatography. If there’s an excessively high leakage, it may lead to decreased quality and contamination of pharmaceutically active substances to be purified. This way, separating the sample from this ligand impurity becomes imperative via expensive downstream processing. Changes in the pH can actively help in preventing ligand leakage. It works by possibly stopping the interaction between the antibody and protein-ligand.
Ligand Leaching and Degradation
It is vital to take the utmost care while handling the resin to minimize ligand degradation. Since most of the ligands used in affinity chromatography are proteins (i.e., antibodies), they require specific temperature conditions to maintain longevity. If not so, the ligands will leach into the elution buffer over time, resulting in decreased column efficiency.
For example, most proteins are stable at 4° Celsius or 39.2° Fahrenheit and degrade at higher temperatures. It is an adverse consequence for purification purposes, but the life of the resin gets severely affected as a consequence of ligand degradation. It is because the resin is helpful until it contains the active ligand, and when the ligand is inactivated or lost, the solid support also becomes useless.
You can overcome this disadvantage through proper column maintenance, such as by regular regeneration or storing the affinity columns in suitable conditions. Moreover, researchers can look for advanced immobilization techniques to improve ligand stability.
Metal-ion Transfer and Leakage
Metal ion affinity chromatography utilizes metal ions bound to the resin. It often leads to the transfer of metal ions to the protein being purified either directly or via leakage in the elusion steps. This way, further purification of the final product becomes a requisite, demanding some extra effort afterward.
Even this problem can be overcome by increasing the dentation number of the chelator, which ultimately increases molecule affinity and reduces the unwanted leakage from the column. Moreover, you can use metal-free chelating columns with strong adsorbents, such as TED.
Column Issue
Sometimes, the affinity columns fail to regenerate, so they cannot be reused. It ultimately increases the resource consumption and overall cost of the chromatography technique.
Researchers should look for different column regeneration methods or consider using disposable pre-packed columns that do not need regeneration.
Limited Scalability and Lifetime
Affinity chromatography presents the limited lifetime of the resins used when the ligands are antibodies. Other ways their lives and scalability are affected are the reasons already discussed, such as ligand leakage and degradation. It is why this process is challenging to scale up for large-scale purification.
Researchers can utilize continuous chromatography techniques besides affinity chromatography to increase overall production. Moreover, add other purification methods, such as protein-A affinity chromatography.
Multiple Purification Steps
The affinity chromatography process sometimes demands multiple purification steps that can further make it complex. This complexity can lead to ligand dissociation, protein denaturation, and other purification challenges.
To overcome this issue, the researcher can pre-plan the entire purification workflow. Moreover, you can consider using multimodal advanced resins to make the process simple and effective. For example, combining ion exchange and affinity chromatography in a single step.
Conclusion
Affinity chromatography is a powerful technique known for the purification of biomolecules in biochemistry and molecular biology; however, you cannot ignore its disadvantages. Researchers must carefully consider the limitations of affinity chromatography when selecting a purification method for their target molecules. To get the most of productivity, get a comprehensive knowledge of this chromatography type through proper experimental design, optimization, and the use of complementary chromatographic techniques.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.

Thank you so much, Jeannie! Your lovely post helped me a lot to provide my presentation for next week!
Could I please get to know the references?
Thank you again.