More people became familiar with the word ‘antibodies’ after the outbreak of Covid infectious cases in 2020. The plasma of coronavirus-infected individuals, containing antibodies, was used to treat and alleviate the symptoms in patients. It saved the time required by the body to produce antibodies against the virus.
However, antibodies are not restricted to only Covid-19 or dengue fever patients. They are produced when the body interacts with any antigen or pathogen that may harm you. Different types of antigens stimulate the production of specific antibodies to protect your body.
Let us tell you everything about antibodies, types of antibodies, and their functions.
What are Antibodies?
Antibodies are the proteins produced by B lymphocytes of the immune system, on exposure to a foreign entity, that strengthens the immunity. They are produced on interaction with a foreign particle and stay in your body to fight them the next time. These antibodies detect unique antigens on the molecules entering the body. In case of expected harm, they attack the antigen and kill it before producing an injurious effect.
Structure of Antibodies
Specific antibodies attack different antigens, yet the basic structure of all antibodies is the same. They are heavy globular proteins consisting of two identical light and two identical heavy chains. The light chain in the structure is denoted by ‘L’ and consists of around 22,000 Da, whereas the heavy chain (H) comprises 50,000 Da. The polypeptide chains are connected using disulfide bonds and may have over 150 kDa.
The light and heavy chains in the antibody structure may also be of various kinds. Light chains are known as kappa (κ) and lambda (λ). Alternatively, heavy chains are indicated by α, δ, ε, γ, and μ.
Research has shown that there are over 200 types of Light (L) chains and around 10,530 types of Heavy (H) chains. When combined in different patterns, they can produce more than 2,100,000 antibodies.
The production of this large number of antibody combinations is attributed to gene restructuring. The genes and alleles in the L chain have the JL and VL gene regions, whereas the H chain has VH, JH, and DH regions. One type of gene from varying gene fragments contributes to assembling the gene regions and produces a new antibody relating to a particular antigen.
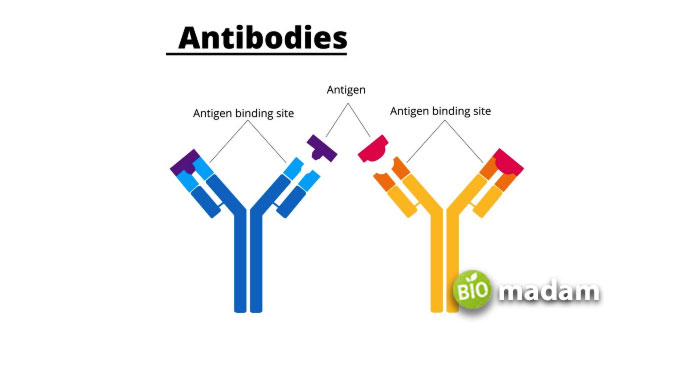
The regions on antibodies can further be categorized as constant and variable regions. The constant region comprises the FC (Fragment Crystallization) region that binds FC receptors on macrophages, WBCs, and killer cells to initiate an immune reaction.
On the other hand, the variable region determines the antigenic specificity of the antibody. It contains the FAB (Fragment Antigen Binding) region that binds to the specific antigen. Each antibody may bind to two epitopes on antigens at one time.
Production of Antibodies
The antibodies are produced in an individual’s body from birth and continue to develop throughout their lifetime. The epitopes influence the production of antibodies on the antigen. While most antibodies are produced specifically to a foreign body entering the body, some do not need stimulation.
B cells use constant-region classes to produce particular immunoglobulins. Sometimes you may be able to find out antibodies in an animal’s blood that they have never been exposed to.
IgM is a widely produced antibody besides IgG, IgE, and IgA. IgA and IgG respond to repeated interaction with infections from a specific pathogen.
Types of Antibodies
Antibodies are widely divided into five types of immunoglobulins depending on their H chain. Changes in the H chain provide specific characteristics to each type of antibody to kill different antigens.
Immunoglobulin M (IgM)
Immunoglobulin is among the first antibodies the immune system produces in case of an infection. They account for 5% of the total antibodies in the body. The population of IgM rises immediately upon interaction with a viral or bacterial infection and continues to fight until IgG takes over. B cells produce IgM antibodies and stimulate action by other immunoglobulins during infection. They are also involved in repetitive fights against the same organism on re-exposure. The memory B cells remember the antigens and direct the antibodies to get rid of the antigen.
IgM is composed of identical subunit polymers, with the pentameric form being the most common. Disulfide bonds join the antibody units in the pentameric form with ten antigen binding sites. Other forms of IgM include the monomeric form and secretory IgM.
Immunoglobulin G (IgG)
Immunoglobulin is the most abundant type of immunoglobulin found in the body. It accounts for around 75% of the total immunoglobulins in the human blood. Immunoglobulin G can be further classified into IgG1, IgG2, IgG3, and IgG4. Proteins trigger the production of IgG1 and IgG2, whereas IgG3 and IgG4 are produced in response to foreign entities.
IgG does not always kill the pathogen themselves by releasing toxins. They might tag the antigen for other immune cells to recognize it. They are also responsible for autoimmune diseases where they trigger an unwanted autoimmune response in the body. The immune system attacks the body’s cells, tissues, and organs.
Immunoglobulin A (IgA)
Immunoglobulin A is produced by B cells and makes up 15% of the total antibodies in the human body. It is found in the mucosal tissues and secreted from the lamina propria. IgG antibodies are typically a part of tears, saliva, and breast milk.
IgA does not work exactly as IgG, yet they also help tag the pathogen for other killer cells. It is the body’s first line of defense and prevents pathogens from sticking to the epithelium tissues. However, IgG may also lead to autoimmune diseases and hypersensitivity reactions in celiac disease patients.
Immunoglobulin D (IgD)
Immunoglobulin D is present in a tiny amount in the human body, accounting for only 0.25% of the total antibodies. It is critical to the initial stages of immune response in the body as they stimulate immunity by attaching to the B-cells. They are often also known as the ‘signaling antibodies.’
IgG promotes the release of IgM to fight diseases and disorders, and prevent antigen attachment to the mucosal lining. They also contribute to B cell activation, maturation, maintenance, and silencing. Despite their role in B-cell antigen reception and promotion of immune response, their further involvement in immunity is unknown.
Immunoglobulin E (IgE)
Immunoglobulin E is also not a widely distributed antibody and has a serum concentration 10,000 times less than IgG. Lymphoid tissues produce IgE near the allergen presence leading to a cascade of events. They also protect the body from parasites and protozoa compared to other immunoglobulins.
IgE is primarily responsible for allergic reactions in the mucosal membrane, lungs, and skin. The first and foremost step of an allergic reaction is the breakdown of mast cells and basophils, which release histamine into the bloodstream. Histamine contributes to the signs and symptoms of allergy.

Camelid Antibodies
Besides the five fundamental types of antibodies, camelid antibodies are also present in animals. They were first discovered in 1989 and do not contain light chains. Camelid antibodies are derived from the Camelidae family of mammals, including camels, llamas, and alpacas. These antibodies are also known as nanobodies and comprise only heavy chains in their structure.
Camelid antibodies are smaller in size than other immunoglobulins and possess low immunogenicity, high affinity, and good solubility. They contain a single variable domain and are widely used in antibody-based therapies.
Immunoglobulin Switching
Exposure to specific antigens activates B cells that produce ID and IgM to kill the antigens. The mature B cells differentiate into other cells and contribute to producing other immunoglobulins besides IgM and IgD. This process is known as immunoglobulin class switching, facilitated by B cell and T cell factors.
What is Antibody Testing?
Antibody testing helps detect diseases a person has previously suffered from. Antigens and antibodies are interlinked as every antibody responds to a particular antigen, Thus, immunoglobulins may help find specific antigens. The presence of specific antibodies and antibody levels can indicate the presence of diseases. Antibody testing is also known as serology and has been widely used in Covid-19 testing globally. Some other virus or bacteria-induced infections and diseases, detected through antibody testing includes:
- H. Pylori
- Polio
- Tetanus
- Viral Hepatitis
- Influenza
- Diphtheria
- Malaria

The Bottom Line
Antibodies make up the body’s defense system against pathogens and other antigens to protect you against diseases. They are produced on interaction with unique antigens to fight them. The five basic types of antibodies in the human body are IgM, IgG, IgA, IgD, and IgE. They comprise two heavy chains and two light chains. Some immunoglobulins remember the antigens and bind to the epitopes on repeated exposure. Their specific composition allows them to attach particular epitopes to antigens.
FAQs
Which is the largest antibody?
Immunoglobulin M is the largest antibody that the human body synthesizes and releases in response to an antigen. However, they are not present in high amounts in the body and account for only 5% of all immunoglobulins. It is the first antibody that the body produces on interaction with an antigen.
What is IgG positive?
IgG positive antibody test shows that the infection occurred a few weeks or months back, and you may have recovered by now. IgG represents immunity to a virus or bacteria your body interacted with in the past.
What happens if IgM is high?
A higher IgM indicates your susceptibility to repeated infections. The immune system might be unable to produce a significant response to pathogens or cancer cells. High IgM levels may also indicate an autoimmune disease like psoriatic arthritis, rheumatoid arthritis or SLE.
How many antibodies do humans have?
Humans may have around 10 billion antibodies produced by different light chain and heavy chain combinations. All different types of antibodies bind to a specific epitope on an antigen to produce an effective immune response.

Anna has completed her degree in Pharmacy from the University of Hawaii. She is serving as a research assistant in a pharmaceutical company. She had a great interest in writing blogs, traveling to different parts of the US, and trying delicious recipes in her spare time.