Energy comes in various forms, including one of the most impressive heat forms. Heat is not only responsible for holding life changes but also aids in food preparation. The distinctive heat properties are the key to different research fields. For instance, heat conductors are applicable to constructing large heaters in buildings by transferring heat.
Different Types of Heat Transfer
Usually, heat picks up three significant phenomena for processing, including convection, conduction, and radiation. Gold is one of the good heat conductors that follow the conduction process. Similarly, there are many practical examples of heat conduction. If you hold a chocolate bar in your hand, it will eventually melt due to heat transfer from your hand to the bar. Moreover, placing a metal spoon on a flame of fire will convert it red hot, again because of the conduction of heat.
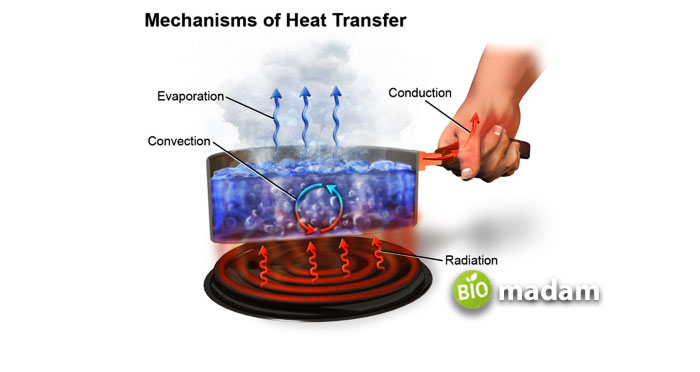
General Method of Heat Transfer Through a Substance (Metal)
Before understanding the nature of gold as a heat conductor, we should know the general phenomenon behind heat transfer. Whenever heat passes through a substance, it sets a temperature difference from one phase to another. It means the substance with more heat content will transfer it towards the one with lesser heat concentration. The process goes on until an equilibrium state is achieved.
Ever wondered, why is metal a good conductor? These are largely considered good conductors of heat and electricity because of the “sea of free electrons” present in them, One such example is silver, which is a good conductor of heat as well as electricity. Both atoms and molecules can work as conductors due to their electrical orbitals. When these orbitals acquire some energy, they form “bands” which are usually the valence bands and conduction bands. A metal, such as gold, conducts heat through it when these bands overlap. We know that gold has a single valence electron, which is quite easy to remove during conduction. That is why it conveniently flaps and conducts heat through it!
Is Gold a Good Heat Conductor?
Before stepping toward explaining the nature of gold, we should know about the nature of all metals. It’s so easy to describe a metal concerning its electrons. Their valence shell has free electrons far away from the nucleus to carry heat and electricity. They are arranged in a delocalized structure with cationic nature for conductivity. That is why metals are good conductors of heat as compared to non-metals that are generally insulators.
Now talking about gold as a good metal conductor, it is naturally very malleable and ductile, leading to its best heat conduction. Despite silver being the best in thermal conductivity, industries usually prefer gold. The reason behind this is, that it has maximum resistance to corrosion. It is another primary cause why gold is used in making durable electrical connectors. Since you can easily mold the nature of gold, it acts as a good heat conductor.
What Factors Affect Heat Conductivity?
If we generally observe, various factors can affect heat conductivity. Let’s discuss the main out of them!
Nature of Substance
Whatever material you are using for heat conduction highly specifies its function. The more the substance has conductivity, the more it will transfer heat. One of the best examples is gold which is a metal. On the other hand, non-metals are poor conductors or insulators of heat and electricity.
Temperature Gradient
The increase or decrease in the surrounding temperature affects a substance’s thermal conductivity. It indirectly depends on the nature of a material. So according to the chemistry of any material and with the rise in temperature, its conductivity changes.
Area of the Material
Thermal conductivity is more enhanced in materials having round openings than the ones with hollow shapes or C-sections. It means the area or the cross-sectional type of a substance also affects its heat conductivity.
Length of the Material
A material having a shorter length will comparatively pass heat more quickly than one with a longer length. It is just like the cross-sectional area, affecting thermal conductivity.
Bottom Line
We went through an entire discussion on the structure of metals and the factors that affect heat conductivity to eliminate your confusion. Now you must understand that gold is, without a doubt, a good conductor of heat. It not only shows its malleability and ductility but also never smudges. All these unique properties make it a perfect fit to be used by several industries utilizing the thermal conductivity phenomenon.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.

