Silver is a metal that is comparatively soft in nature and gives a shiny surface. Our primary goal is to reach whether silver is a good conductor of heat or not, but before that, wouldn’t it be perfect if we revise our concepts?
We know that, in the periodic table, there are numerous elements with defined sections, where one such is a section of metals. But, why is metal a good conductor? These are considered the best conductors of heat and electricity because of their valence shell electrons. Any atom, molecule, or compound capable of giving its outer shell electrons easily is a good conductor! Talking metals, have one, two, or even three outer shell electrons that fall out to give a positive charge to the respective element. In this article, we will discuss heat transfer and thermal conductivity to conclude how silver is a good conductor of it.
Explain the Property of Thermodynamics
There are different laws of thermodynamics, with the second one stating:
“The content of heat always passes from a substance with increased temperature to the one with decreased temperature.”
It means atoms with higher energy will continually shift to those with lower energy to attain equilibrium. This equilibrium state achieved due to heat is called thermal equilibrium. Heat usually transfers through different mechanisms, including conduction, radiation, and convection. As we are only concerned with the conduction phenomenon, we will proceed with the concept of heat transfer by conduction.
Explain the Transfer of Heat by Conduction
We decided to walk through the conduction process with a general understanding of thermodynamics. It explains that heat passes from an atom of higher energy to an atom of lower energy following vibration. All the high-energy neighboring atoms vibrate and collide with one another to produce heat. This heat, through electrons, automatically reaches the points at lower energy and runs the phenomenon of conduction. Solids show good conductivity than gases and liquids, with metals being the best of all conductors!
Reasons Behind Good Heat Conductivity of Metals
Before apprehending the reasons behind metals as good conductors, understand how they function. There is a cloud of extra electrons in all metals, having the most trivial influence on their nucleus, and are given out easily. All these free/extra electrons can conduct heat and electricity and displays a positive charge on the respective metal.
When talking specifically about thermal conductivity, there appear two fundamental reasons:
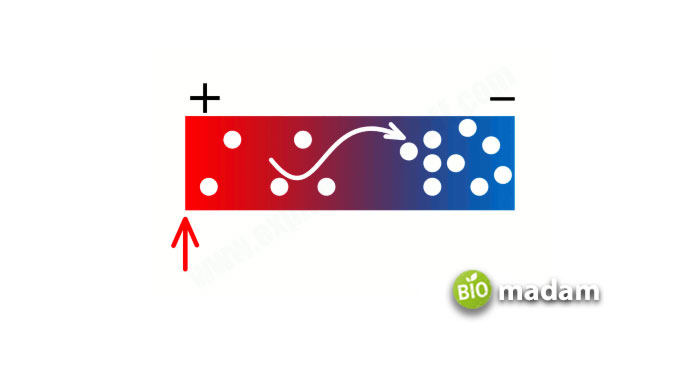
- All metal ions have tight packing in the form of a lattice, which produces vibration.
- The free electrons that are delocalized possess sufficient kinetic energy for the movement and conduction of heat.
Examples of Heat Conductors
We come across several heat conductors daily, but as we review metals and their conductivity, let’s see a few of them. Some common examples of metals as heat conductors are:
- Silver
- Aluminum
- Brass
- Steel
- Gold
- Iron
- Copper
- Bronze, etc.
Silver – A Good Conductor of Heat
After all the above discussion, it is obvious that silver, being a metal, is a good conductor of heat. It has an adequate quantity of moving electrons that are conveniently transmitted to produce a positive charge. Silver conducts heat through it by the phenomenon that:
“The more the loose electrons, the higher is its heat conductivity.”
Final Discussion
After giving a complete overview, we now hope that the concept behind why silver is a good conductor of heat is cleared. Silver, along with other metals, is highly active to give its valence shell electrons Hence, it is also a good conductor of electricity. Although this property makes it stand in the list of good conductors, we usually do not apply it practically. The reason lies in it being very expensive! Silver is particularly used in circuit boards or satellites.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.

