A student should always be well aware of making a solution when working in chemistry or biochemistry laboratories in institutes. Today we appeared to give a brief concept of molecular mass, molar mass, molar volume, molarity, and molality. Furthermore, you should know how to make a molar or a molal solution. When you grasp all these terms and their uses, you can easily make every type of solution or suspension, be it molar or molal! Most of these lab techniques are observed under analytical chemistry, one of the five primary branches of chemistry
What is Molarity?
The number of moles of a substance dissolved per dm3 of the solution is called molarity.
Symbol of Molarity: M
Formula
Molarity = no. of mass of solute/volume of solution dm3
Molar Solution
When one gram mole of solute dissolves in 1 dm3 solution, it forms are one molar solution.
Example
Calculate the molarity of a solution containing 20.7 g of K2CO3 dissolved in 500 cm3 of the given solution?
Solution
Mass of K2CO3 = 20.7 g
Molar mass of K2CO3 = 138g/mole
Volume of solution = 500 cm3 = 0.5 dm3 (500/1000 = 0.5)
(For example: 1 dm3 = 1000 cm3, another important rule is the definition 1 liter = 1 dm3)
Molarity = 20.7g/138g/mole * 1/ 0.5 dm3
Answer = 0.3mol/dm3
What is Standard Solution??
A very significant concept relating to practicing is the standard solution, so you should know about it. A standard solution is the solution of known molarity.
Example
0.1 molar NaOH
0.05 molar HCl
What is Molality?
The number of moles of a substance dissolved per 1g of the solution is called molality.
OR
The number of moles of solute in one gram of solvent.
Symbol of Molarity: m
Formula
Molality = no. of mass of solute/volume of solution in kg
Example
What is the molality of a solution prepared by dissolving 3g of toluene in 250g benzene?
Solution
Mass of toluene = 3g
Mass of benzene = 250g = 0.25kg
Conversion in kg = 250/1000 = 0.25kg
Molar mass of toluene = 92 g/mole
Molality = (mass of substance/molar mass of substance) * 1/volume of solution in kg
= 3g/92g/mol * 1/0.25
Answer = 0.13 mol/kg
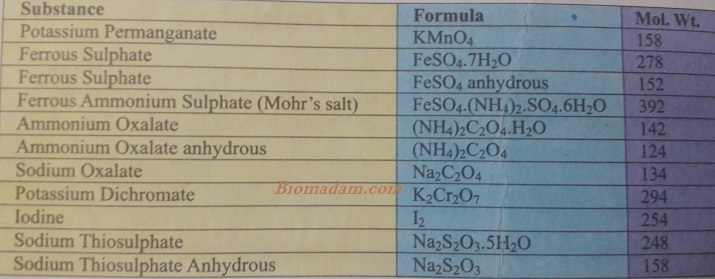
Some important salts with their molecular formulas and weight.
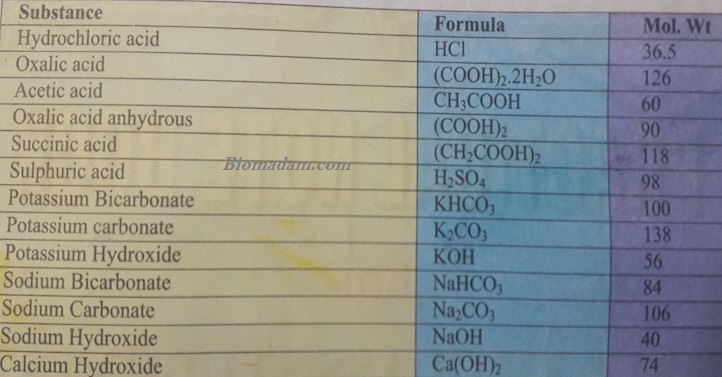
Some essential acids with concentrations, so you can prepare solutions according to the following weights.
You can make a 0.1 M solution of the above salts by using the complete data.
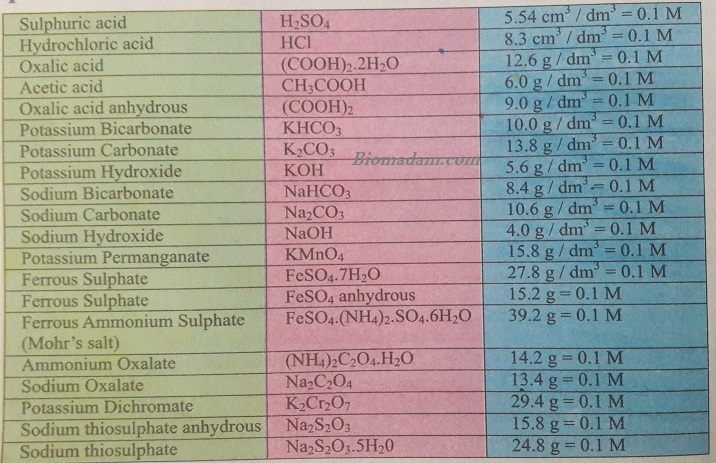
Hence, when you apprehend all about these things, you can easily make each type of solution.
Following are Some Solutions and Preparation of Standard Curves
How to Prepare a 100 ml Solution of 1 Molar Glucose?
Apparatus
Beaker, stirrer, weight balance, glucose, distilled water
Calculations
Molar mass of glucose (C6H12O6) = 6(12) +12 + 6(16) = 180 g
Amount of glucose required = [molar mass/1000] * volume required
Amount of glucose required = [180/1000] * 100 = 18 g
Procedure
- Weigh 18g of glucose by using a weight machine.
- Take a beaker and add glucose to it.
- Add some amount of distilled water.
- Dissolve it, then make the volume up to 100ml.
- Store in an appropriate container.
How Can You Make a 4% Solution of Glucose?
Principle
Percentage composition is expressed as the solute and solvent amounts dissolved per 100 parts by the solution.
Apparatus
Beaker, stirrer, electric weight machine, distilled water, glucose
Procedure
- Weight 4 g of glucose by using electric weight balance.
- Add this calculated glucose in a beaker.
- Add 30-40 ml of distilled or boiled water to dissolve the glucose.
- Stir the solution to dissolve the glucose.
- Add more water to make the volume upto100ml.
Note: Gently heat the solution if the solute does not dissolve properly.
Prepare Phosphate Buffer of pH 6.0, Molarity 0.05, and Volume 100 ml?
Buffer is a solution of weekly dissociated acid and its conjugate base, and vice versa.
Apparatus
Beaker, weight balance, spatula, stirrer, KH2PO4, K2HPO4, distilled water, KOH or HCl solution, pH meter
Calculations
pH. = pKa + log[S] / [A] …. (1)
By putting the values in equation (1):
6.0 = 7.2 + log [S] / [A]
6.0 – 7.2 = log [S] / [A]
-1.2 = log [S] / [A]
1.2 = log [A] / [S]
Antilog 1.2 = log [A] / [S]
15.55 = [A] / [S]
15.55 / 1 = [A] / [S]
Number of moles of acid = [15.55 / 16.55] × 0.05 = 0.046 M
Moles of salt = [1 / 16.55] × 0.05 = 0.003 M
Amount of acid required = [molecular mass / 1000] × moles × volume required
Amount of acid required= [136 / 1000] × 0.046 × 100 = 0.652g
Amount of salt = [174 / 1000] × 0.003 × 100 = 0.052g
Procedure
- Take a beaker and add weighed amount of acid and salt in it.
- Add distilled water to make the volume approximately 90 ml and mix thoroughly.
- Check the pH of a solution by pH meter.
- Adjust the pH with corresponding acid or conjugate base if desired.
- Make the volume up to 100 ml and store in an appropriate container.
How will you Make the Standard Curve of Glucose?
Procedure
- Weight 0.1g glucose and dissolve it to make 100 ml.
- Take 100, 200, 300, 400, 600 µL separately and make 10 ml with distilled water.
- Take 1 ml from this stock solution in a separate test tube add 1 ml phosphate buffer of pH 6.8.
- Add 3 ml DNS and boil it in the water bath for 5 min.
- Cool it to room temperature, then measure its absorbance at 550 nm.
Calculations
|
Sr. No |
Standard glucose solution (ul) |
H2O (ml) |
Total volume (ml)* |
Glucose concentration (ug/ml) |
Buffer (ml) |
DNS reagent (ml) |
Absorbance at 550 nm |
|
1 |
100 |
9.9 |
10 |
10 |
1 |
3 |
0.03 |
|
2 |
200 |
9.8 |
10 |
20 |
1 |
3 |
0.10 |
|
3 |
300 |
8.7 |
10 |
30 |
1 |
3 |
0.31 |
|
4 |
400 |
9.6 |
10 |
40 |
1 |
3 |
0.75 |
|
5 |
600 |
9.4 |
10 |
50 |
1 |
3 |
1.2 |
|
Blank |
000 |
10.0 |
10 |
00 |
1 |
3 |
00 |
Standard Curve Graph Plot
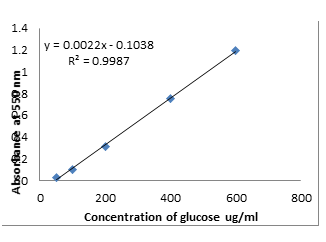
Concentration of Glucose x = y + 0.1038
= 0.0022
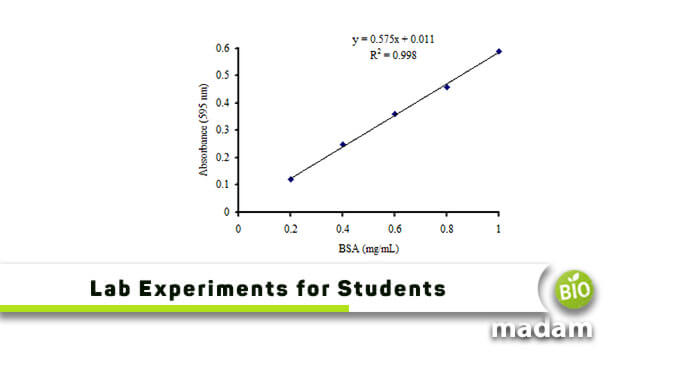
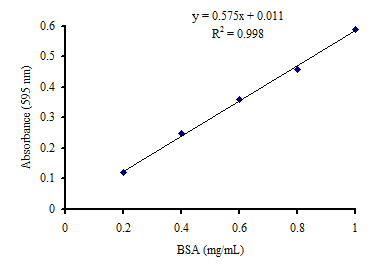
Find Out Standard Curve of BSA for Protein Estimation?
Bradford Assay
Material
Bovine serum albumin, Bradford reagent, distilled water
Equipment
Spectrophotometer and test tubes
Procedure
- Take 0.1 g bovine serum albumin and dissolve it in distilled water to make 100 ml (1 mg/ml).
- Take 20, 40, 60, 80, 100 µl, separately and add water to make final volume up to 100 µl.
- Add one ml of Bradford reagent and mix to develop color.
- Take absorbance at 595 nm.
- Prepare blank by adding water instead of BSA standard solution.
- Make a standard curve to quantify the protein content of unknown samples
Below is a standard curve of bovine serum albumin for the https://www.biomadam.com/molecules-vs-compounddetermination of total protein content.
After reading these calculations, you will be able to understand and know some following questions.
Catch a Glimpse on the Following Questions
How much does one Glucose molecule weigh?
One molecule of glucose has a formula C6H12O6, which weighs 180 grams.
How many grams are in a mole of Glucose?
There are 180 grams in one mole of glucose.
What does C6H12O6 stand for?
The above formula stands for Glucose.
How to Make a Molar Solution?
You should always know about the quantity of a substance before making its molar solution. For example, 0.1 or 0.01 molar of sodium chloride.
Example
You want to make a 0.1 molar solution of NaCl.
NaCl has mol wt. equal to 58g/mol
Weigh 5.8 g NaCl and dissolve it in one-liter water. Now, this is one molar solution. The same method goes for other concentrations.
Note: This method applies only to solid solutions

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.