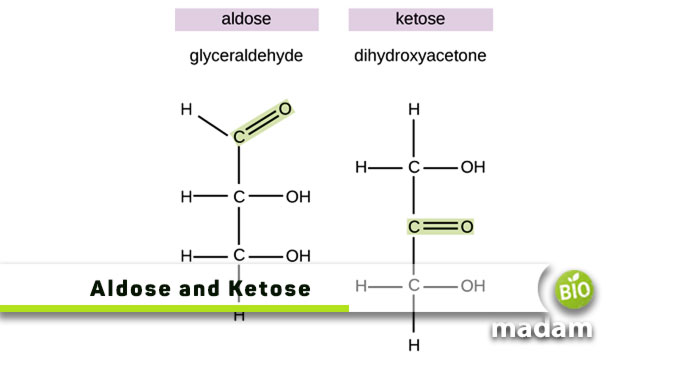
Our body requires different macro and micronutrients to carry out several activities. Aldoses and ketoses are carbohydrates that are essential in total calories and provide energy to the body cells. Both are monosaccharide sugars, but differ in their structure. Aldoses contain aldehyde groups while Ketoses have ketone groups. The difference in their structure contributes to unique functions in prokaryotic and eukaryotic organisms.
Keep reading to know all the differences between Aldoses and Ketoses.
Comparison Table
| Characteristics | Aldose | Ketose |
| Essential Functional Group | Aldehyde | Ketone |
| Source | Plants | Processed foods |
| Seliwanoff’s Color | Light pink | Cherry red |
| Chiral Centers | More | Less |
| Examples | Glucose, ribose erythrose, galactose | Sorbose, xylulose ribulose, fructose |
What is Aldose?
Monosaccharides that contain an aldehyde group are known as aldoses. Aldoses are a particular class of monosaccharides in which the aldehyde functional group attaches to the compound’s carbon chain as the primary functional group. The (-CH=O) aldehyde group is a reactive chemical group. Sugar molecules with two carbon atoms and one aldehyde group are the most basic type of aldose. Aldoses are acyclic monosaccharides that contain an aldehyde in the absence of the hemiacetal form.
Chemical Formula
Aldose exists as optical isomers of the D (dextrorotatory) and L (levorotatory) types. It has the general formula Cn (H2O)n. where ‘n’ represents the carbon atoms that constitute the monosaccharide compound’s backbone.
Structure of Aldose
Simple, pure sugar is an aldose. The majority of aldose molecules have a six-ring shape and a biological structure. There are multiple ways to describe the structure of carbohydrates, just like it is for proteins and other nutrients. German scientist Emil Fischer, the winner of the Nobel Prize, invented the Fischer Projection Formula, one of the most popular methods.
When the alcohol group (-OH) is on the right side of the Fischer projection, it falls under the D-aldose classification, and when it is on the left side, it belongs to an L-aldose. In general, D-aldoses cause biological receptors to become more sensitive than L-aldoses.
Aldose Classification
Depending on how many carbons are in the main chain, aldose has divisions into the following groups.
Trioses: Triose is the name for an aldose with three carbons. The triose glyceraldehyde, the most basic aldose, serves as an illustration.
Tetroses: A tetrose is a four-carbon carbohydrate. Threose and erythrose are two examples of tetrose aldoses.
Pentoses: A pentose is a five-carbon carbohydrate. Ribose is a well-known five-carbon aldose.
Hexose: Hexoses are six carbon carbohydrates. Glucose is one of the most well-known aldoses and an example of aldohexoses.
Aldose Functions
Glucose: The primary energy source for our bodies is glucose, the main sugar found in the blood. It is present in standard dietary products, including milk, fruits, bread, potatoes, and other foods similar to tubers. A high amount of glucose in the body can result in hyperglycemia, whereas its low blood quantity leads to hypoglycemia.
Ribose: Another crucial component of living things is ribose. It plays a role in developing the backbones of DNA and RNA, which are vital parts of biological systems.
Galactose: A monosaccharide, quite seen in its free form, is a crucial part of the milk sugar type (lactose). In the absence of food, it might serve as a source of energy for our bodies.
Arabinose: Almost every plant we observe growing on the land contains a significant amount of this sugar in its cell wall.
Advantages
- Proper functioning of several body tissues and organs depends on the aldose reductase response.
- The most crucial sugar in a person’s body is glucose, also known as an aldohexose. It functions as cellular fuel.
- The body uses ATP, which is a product of glyceraldehyde, to carry out various metabolic processes like muscle contraction.
Disadvantages
- The galactose in milk and dairy products can lead to diarrhea and digestive issues.
- Diabetes and other severe health hazards might result from high glucose levels.
What is Ketose?
Ketose is a carbon-based sugar molecule (monosaccharide) with cyclic and open-chain structures. The monosaccharide ketose has a ketone group attached to its carbon backbone.
The ratio of ketone groups to sugar molecules is typically one. Chemically speaking, the ketone group (-C=O) is reactive. The sugar molecule with three carbon atoms, one of which has a ketone group in the middle, is the most basic type of ketose. In the carbon chain, ketoses have several stereogenic centers, like aldoses. Due to their ability to tautomerize into aldoses via an enediol intermediate and the subsequent ability to oxidize the resultant aldehyde group, all monosaccharide ketoses are reducing sugars.
Chemical Formula
The formula for ketose is Cn (H2O)n, where ‘n’ represents the number of carbon atoms that constitute the backbone. This formula is the same as that for aldose.
Structure of Ketose
Similar to aldoses, Fischer projection equations represent the chemical structures of ketoses. The carbonyl functional group in ketoses has no relation to a carbon atom at either of the chain’s two ends. As a result, the carbonyl atom has adhesion to two carbon atoms (through two single bonds) and one oxygen atom, totaling three atoms (via a double bond).
The primary alcohol groups, found at each end of the backbone, have a connection to the remaining carbon atoms by hydroxyl groups.
Ketose Classification
Depending on how many carbons are in the main chain, ketoses have divisions into the following groups, like aldoses.
Triose are molecules with three carbon atoms.
Tetrose ketosis contain four carbon atoms in their structure
Pentoses are five-carbon containing molecules.
Ketose Functions
Fructose: Most often present in many natural fruits, some plant foods like honey, and some vegetables, fructose is a six-carbon ketose.
Dihydroxyacetone (DHA): DHA is the most basic form of the ketose family and contains three carbons. As a significant component of sunless tanning, it has extensive participation as a decorative element.
Tagatose: This ketose results from cooking milk. Numerous dental products also contain it. Furthermore, the artificial sweetener industry is using it.
Advantages
- One of the ketoses, fructose, aids in improving glucose metabolism.
- Ketoses are the simplest carbohydrates that serve as the building blocks for nucleic acids.
- To increase the body’s metabolism, many ketoses are crucial.
Disadvantages
- Inflammation and other significant medical issues might result from consuming too much fructose.
- Fructose can raise the body’s harmful cholesterol levels, which leads to fat storage.
- Aldose and ketose monosaccharides are examples of simple carbs.
- The amount of carbon items determines the subcategories for both sugars.
- Both have the same empirical chemical formula.
Difference Between Aldose and Ketose
Definition
Aldose
An aldose is a monosaccharide carbohydrate with a carbonyl group at the endmost carbon atom.
Ketose
Ketoses, on the other hand, are sugars containing a ketone group per molecule in its acyclic form.
Functional Group
Aldose
Aldose has a single aldehyde group.
Ketose
On the contrary, ketose has one ketone group per molecule.
Source
Aldose
Plants are the primary source of aldose.
Ketose
Whereas, ketoses are primarily obtained from processed foods.
Isomerization
Aldose
Aldose may transform to ketose.
Ketose
At the same time, ketoses isomerize into aldose.
Seliwanoff’s Test
Aldose
Aldose displays light pink color in Seliwanoof’s test.
Ketose
Meanwhile, ketose exhibits cherry red color.
Chiral Centers
Aldose
Aldose has more chiral centers, e.g., glucose has four chiral centers.
Ketose
Conversely, ketose has a lesser number of chiral centers. E.g., fructose has three chiral centers.
Examples
Aldose
Glucose, galactose, erythrose, glycolaldehyde, and glyceraldehyde are aldoses.
Ketose
Ketoses include sorbose, nonoses, pentoses, tetroses, fructose, and xylulose.
The Bottom Line
Aldose and ketoses are important monosaccharides in the body to perform various functions. Plants are an adequate source of aldoses as they produce glucose through photosynthesis. On the contrary, you can obtain ketoses from processed food. Both of them have the ability to isomerize into each other. Glucose, galactose, and erythrose are examples of glucose while sorbose and fructose are ketoses.
FAQs
What is the difference between aldose and aldehyde?
Aldehyde refers to a chemically reactive group. The molecule containing two aldehyde groups is known as an aldose. Aldoses may have multiple forms with the basic formula Cn (H2O)n.
What is the difference between an aldose sugar and a ketose sugar?
Aldose sugars have an aldehyde group while ketose sugars have a ketone group. Moreover, one is a linear while the other is a ring form.
What is aldose and ketose formula?
Ketoses and aldoses are monosaccharides with a carbonyl group at the end. Thus, they have the same formula: Cn (H2O)n. ‘n’ represents the number of carbon atoms forming the backbone of the molecule or compound.

Hello, I would like to introduce myself to you! I am Chelsea Rogers, an experienced blog writer for science articles, holding an MPhil degree. My enthusiasm to grab the best knowledge, let it relate to botany, zoology, or any other science branch. Read my articles & let me wait for your words s in the comment section.