The electric current is an important physical property of any substance that differentiates conductors from insulators. Electrodes contribute to the generation of electric current to enable conductivity. The electrode can be negative or positive. The cathode is the negative electrode that releases electrons, while the anode is the positive electrode that acquires electrons in an electrolytic cell. Let’s tell you how both of them work and all the differences between anode and cathode in detail.
Comparison Table
| Factors | Anode | Cathode |
| Meaning | Way upwards | Way downwards |
| Definition | Positive electrode | Negative electrode |
| Electricity Flow | Inwards | Outwards |
| Associated Rxn. | Oxidation | Reduction |
| Charge in Galvanic Cell | Negative | Positive |
What is an Anode?
The anode is the positively charged electrode of an electrochemical cell that attracts electrons.
“Anode” is a Greek word that means “way upwards.” Although, anodes are positively charged electrodes in electrolytic cells, they act as a negative electrode in a galvanic cell. In simple words, an anode is a point where an oxidation reaction takes place. An anode acts as a positive electrode in an electrochemical reaction because electrochemical reactions use electrical energy to transmit a chemical reaction.
In contrast, in a galvanic cell, the anode acts as a negative electrode as it has a negative ability compared to a galvanic cell’s solution. The anode is a zinc metal in a galvanic cell dipped in a ZnSO4 solution. Anode and cathode also help understand the electric current in other liquids and aqueous solutions.

Properties of Anode
- Anodes act as positive electrodes in electrolytic cells and negative electrodes in galvanic cells
- Anodes work as an efficient oxidizing agent
- Anodes have a good level of electrical conductivity
- They have high coulombic output
- Anodes are stable in nature
- They are low-cost electrodes
What is the Cathode?
A cathode is a negatively charged electron that allows electrons to enter an electric device.
The word “cathode” originated from a Greek word that means “way towards downwards.” Generally, a cathode is an electrode where reduction takes place, which means that the cathode gains electrons from the external circuit and gets reduced. Cathodes are often used in electron microscopes.
In a galvanic cell, a cathode is a copper metal dipped in a CuSO4 solution. A cathode electrode is of two types; a hot cathode and a cold cathode. Hot cathodes are heated by electric current passing through the filament. In comparison, the filament does not heat the cold cathodes. These tubes are used in vacuum tubes, discharge tubes, discharge lamps, etc.

Properties of Cathode
- Cathode works as an efficient, reducing agent
- Remains in control when it comes in contact with an electrolyte
- Helpful when used as a working voltage
- Easy in terms of fabrication
Difference Between Anode and Cathode
Literal Meaning
Anode
Anode comes from a Greek word that means “way upwards.”
Cathode
On the contrary, cathode translates to “way downwards” in its origin word.
Definition
Anode
The anode is the positive electrode in an electrolytic cell that acquires electrons.
Cathode
On the other hand, the cathode is the negative electrode in an electrolytic cell that releases electrons.
Flow of Electricity
Anode
In the anode, electricity flows inside the device.
Cathode
Contrarily, in the cathode, electricity flows outward.
Charge of Electrode
Anode
The anode is mostly on the positive side, depending on the type of cell used during the reaction.
Cathode
At the same time, the cathode typically acts as a negatively charged electrode.
Reaction in Electrolytic Cell
Anode
The anode is the location for oxidation reaction in the electrolytic cell.
Cathode
Conversely, a reduction reaction occurs at a cathode electrode in an electrolytic cell.
Role in a Galvanic Cell
Anode
In a galvanic cell, the anode can act as a cathode. It means that the charge of the anode will turn into a negatively charged electrode.
Cathode
Whereas the cathode can act as an anode in a galvanic cell. This means that the charge of the cathode will turn into a positively charged electrode.
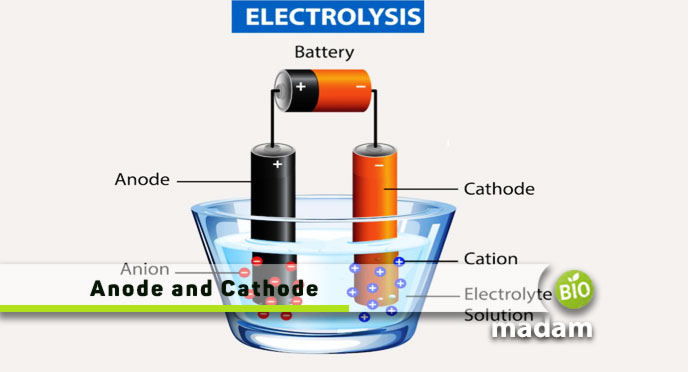
Anode and Cathode in Electrolysis
You can study the electric movement of any solution or suspension using a cathode or anode including aqueous solutions. During the process of electrolysis in a chemical reaction, the change occurs through electricity transmission in the circuit. An electrolytic cell is a device that changes electrical energy into chemical energy. As far as the charge of ions is concerned in an electrolytic cell, an anion is a positively charged, while a cation is a negatively charged ion. However, both cations and anions have opposite roles in general.
For a better understanding of the electrolysis of a cathode and anode, Let’s take the example of the electrolysis of molten NaCl. Before electrolysis, two electrodes are dipped in the solution of NaCl. The anode acts as a positive charge electrode as it is connected to a positive terminal of the battery. At the same time, a cathode acts as a negatively charged electrode when connected to a negative terminal of the battery. The anions will then move toward the anode and release electrons to the anode, and get oxidized. Na+ ions will move towards the cathode when electricity passes. They are reduced to become sodium metal. In contrast, Cl– ions will move toward the anode, where it is oxidized to become sodium metal.
The Bottom Line
Anode and cathode are important electrodes used in electrolytic and galvanic cells. The anode acts as a positive electrode in electrolytic cells and acts as the site of oxidation. On the other hand, the cathode serves as the negative electrode and undergoes reduction. Anodes offer a high level of electrical conductivity, while cathodes are helpful when used as a working voltage. They both contribute to the understanding of electrical conductivity and different lab experiments.
FAQs
Is anode always positive?
The anode is usually positive, but the charge on the anode depends on the type of the cell. The anode is considered negative in a galvanic cell where the cathode is positive.
Why is cathode negative?
In an electrolytic cell, the electrons move away from the anode towards the cathode. Thus, it makes the cathode negative and the anode negative.
How can we identify the anode and cathode?
If you want to distinguish anode from cathode when written in simplified cell notation, the cathode is on the right side, whereas the anode is on the left.

The Biomadam Content Team develops and manages educational and informational articles published on Biomadam. Content is prepared using standard reference materials and follows internal editorial guidelines to ensure clarity and consistency.