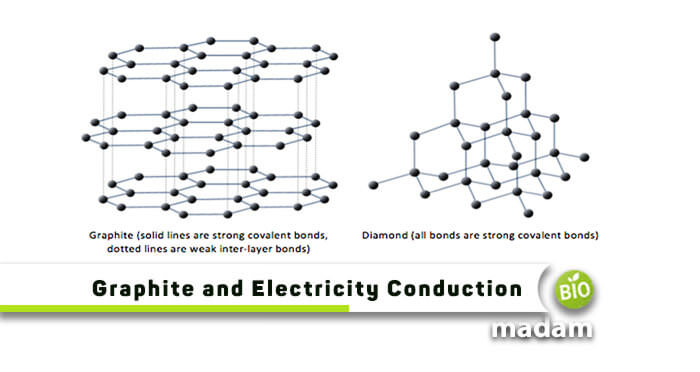
Baffled in thinking if graphite is a good conductor of electricity or not. Before we focus on our primary topic, let us first grab the central knowledge behind conductors and conductivity.
Every material present on Earth has its own characteristics, and so do the smallest particle atoms. If we see our surroundings, everything sequentially starts from the combination of millions of trillions of atoms. An atom consists of protons, neutrons, and electrons. These electrons have the distinctive property of being electric charges and can conduct electricity. Now, what do you understand by conductivity? In simple words, the phenomenon of passing electricity through any material is its conductivity. Science has classified everything present around the globe as superconductors, conductors, and insulators.
Brief Contrast Between Conductors & Insulators
All those materials that can easily allow electricity or heat to travel through them are conductors. A good conductor can be either a metal or a non-metal. Whereas on the other side, insulators can be any substance that restricts electricity from crossing them.
Know About Electrical Conductors
If you have assimilated the above concept, it’d be easy for you to understand electrical conductors. All those materials that can easily engage electricity in them are electric conductors. Moreover, in such substances, the valence electrons are loosely associated with the nucleus, making them good conductors. There is an entire range of electrical conductivity in different elements, having different strengths. A few examples of electrical conductors are as follow:
All these materials are least resistant to charges and easily get attracted, which shows them as good electrical conductors.
What is the Nature of Graphite – A Good Electrical Conductor?
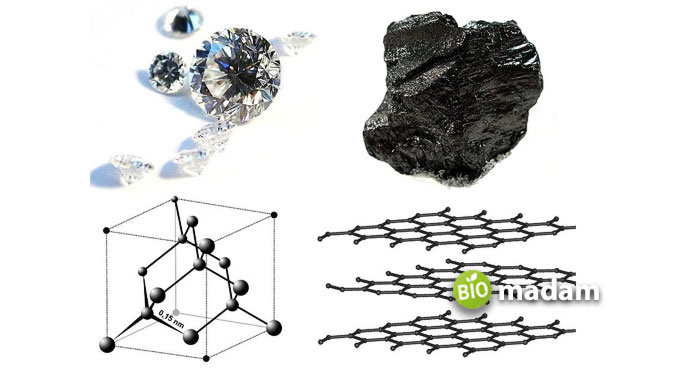
Graphite is another name for carbon with a crystalline structure. It is a non-metal layered substance that is known as a suitable electrical conductor. Most non-metals are not so best in conductivity, but what allows this compound to show conductivity is its structure and nature. The hexagonal shape of graphite promotes it to pass electricity through it. We’ll discuss the phenomenon later in the topic below!
Reason Behind Graphite Being a Good Conductor of Electricity
The central idea behind this substance showing good electrical conductivity is its structure. We should first clear the confusion that graphite is all composed of carbon atoms. We also know that every carbon atom forms four bonds with other elements for its stability. In graphite, each carbon atom is directly engaged with only three other atoms, and the rest is set to spare. The free-floating electrons in graphite enhance their conductivity and allow electrical charges to travel through them.
What Are the Characteristics of a Good Electrical Conductor?
Now that we know why graphite is a good conductor, so let’s have a look at the general properties of electrical conductors.
- Electrons in good conductors can easily flow because of the zero electric fields.
- A conductor will always exhibit its conductivity by passing charged ions or electrons through it.
- It doesn’t matter if it’s metal or non-metal, but free electrons or ions can only float on the surface of a good conductor.
- A conductor presents zero charge density.
- Every conductor appears with its unique characteristics in equilibrium, at equal potential.
Conclude a Statement About Graphite
We mostly have encountered metals as good electrical conductors. But after reading this article, we hope to clear the conflicts regarding graphite and the concept behind why it is a good electrical conductor. Because of its unique conductivity, graphite is widely used as an anode in different industrial matters.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.