A chemist is a scientist who studies and experiments with various processes in chemistry. In broader terms, chemists study matter, its properties, composition, the chemical reactions happening, how these reactions result in new products, and so on.
Below, we have presented the top scientists to celebrate the necessity of chemistry, its sub-fields, and how this field revolutionized and transformed during the last decades. Besides, we have mentioned brief overviews of their achievements and contributions.
Antoine-Laurent de Lavoisier/Antoine Lavoisier

Lavoisier, also known as the father of modern chemistry, was a French chemist. He was born in 1743 and died in 1794. We usually know this scientist for his influences in both the histories of different branches of chemistry and biology. The most significant contributions in chemistry include:
- Oxygen theory
- Presenting the concepts of stoichiometry
- The nomenclature of chemicals
- The chemical revolution
Oxygen Theory
During late 1772, the phenomenon of the combustion of oxygen got Antoine’s attention, which enabled him to make his most significant contribution in the field. Antoine’s first experiments were on phosphorous combustion, which he reported to the academy on 20th October (1772) in the form of a letter. This report stated that when phosphorous burns, it increases in weight. Moreover, the phosphorous combines with a vast air to produce the so-called acid spirit upon burning.
This first report was accompanied later by a second report where he extended his keen observations and conclusions about the experiments. This report also contained the burning of sulfur and phosphorous. He implied that the changes in the burning of sulfur and phosphorous might also apply to all substances that increase their weight after combustion.
Stoichiometric Concepts
Antoine was one of the few researchers who are the pioneers of doing quantitative chemical experiments. In doing so, he used to carefully weigh the reactants and products in closed vessels so that no gas exchange occurs between the reactions and the outside environment. Such experiments let him establish that the total mass of matter during chemical reactions remains the same (in 1774).
Dmitri Mendeleev

He was a Russian chemist and an inventor, best known for formulating the Periodic Law and the periodic table of elements. He was born in 1834 and died in 1907. Also, his farsightedness led him to formulate Periodic Law that was used to correct the properties of already known elements. Moreover, it helped predict the elements that still need discovery.
Discovery of Periodic Table
In 1863, when we had only 56 known elements present, several scientists tried to define the periodicity of these elements. Initially, John Newlands presented the law of octaves, which tried to put the elements periodically because of their atomic weights. Similarly, Lothar Meyer also struggled to arrange the elements periodically according to their valence properties. But each of these had its flaws associated with them.
It was about 1867 when Mendeleev was preparing a chemistry textbook, allowing him to make the periodic table’s most important discovery. The scientist tried to classify the elements based on their chemical properties in his textbook, noticing some patterns. According to Mendeleev himself, it was a dream that showed all the elements perfectly fell into appropriate places as required. He immediately noted on a paper after waking up (except one place, which he corrected later).
After Mendeleev developed the periodic table with all known elements, he published it in a Russian journal. Also, Meyer printed his periodic table within a few months, which was virtually identical to that of the Mendeleev’s. However, Mendeleev’s table distinguished as it accurately predicted germanium, gallium, and scandium properties. Due to Mendeleev’s unmatchable work on the periodic table, we remember him as the Father of the Periodic Table.
Linus Pauling

Linus Carl Pauling – an American chemist, chemical engineer, biochemist, and peace activist – was born in Portland, Oregon – a state of the US. He lived from 1901 to 1904, during which he made spectacular achievements of his career by publishing more than 1200 papers and books. He won two noble prizes – one in 1954 for his work in chemistry and the other in 1962 for his peace activism. Now let us dig a little deeper into his achievements one by one.
Chemical Bond and its Nature
Pauling started working on the nature of chemical bonds in the late 1920s, which led him to publish his research. He was then appointed as a lecturer in chemistry at Cornell University. He delivered a series of lectures on this subject while completing his famous textbook ‘The Nature of Chemical Bond.’ Due to this work, he got his noble prize in chemistry in 1954.
Furthermore, he also introduced the concept of orbital hybridization. Due to this concept, the orbitals are considered to have partaken the effect of each other. For example, the one 2s and three 2p orbitals on carbon are mixed to form four orbitals of equivalent energy in the type known as sp3 hybridization. Other types of hybridizations include sp2 and sp hybridizations with their properties.
He explored another area of chemistry, which is the relationship between ionic and covalent bonding. Ionic bonding is the type of bonding in which electrons are transferred between atoms, whereas in covalent bonding, the electrons are shared between the atoms. Pauling viewed these two bonding types as mere extremes and explained with the help of quantum mechanics that a polar molecule AB can be described as a combination of the wave functions of both ionic and covalent bonding.
He also made great efforts in describing the structure of the aromatic compounds and came up with a resonance theory for them by implementing quantum mechanical approaches to explain them. We all know that Friedrich Kekule best explained the simplest of aromatic compound structures, such as benzene. The alternate double bonds shuffle their positions via rapid interconversion. However, Pauling proposed a superposition of the bonds rather than their rapid interconversion. Also, this phenomenon resembles the concept of hybridizations and polar bonding too.
Ionic Crystals Structures
Pauling also worked on predicting and explaining the structures of the ionic molecules, which he published in 1929 in the form of five rules which are:
- The ratio of cation and anion radii,
- The strength of electrostatic bonds,
- The sharing of corners, edges, and faces of a polyhedron,
- The formation of different cations in the crystal, and
- The parsimony rule.
Work on Biological Molecules
The primary influence on Pauling to work in biology came in the 1930s when the funding priorities shifted more towards biological sciences, which made him take a decisive shift of interest to explore new fields. He interacted with great biologists, such as Thomas Hunt Morgan, Theodosius Dobzhansky, Calvin Bridges, and Alfred Sturtevant.
One of his early works in biology included studying the hemoglobin molecule and its structure changes when it binds with oxygen molecules. This work helped him study the protein structures in more detail. He tried to use X-ray diffraction analyses, but this didn’t prove to be much successful. This technique was far less amenable for crystals of biological molecules.
Pauling, Robert Corey, and Herman Branson correctly proposed the secondary structural elements of proteins – alpha helices and beta sheets. Moreover, they also presented the assumption that the one turn of the helix may contain a non-integer number of amino acids, which we now know is true.
Pauling also proposed the triple helical structure of the DNA, but since this model contained several mistakes, it faced significant criticism and conflict. Moreover, he also studied enzymatic reactions and pointed out that enzymes work by stabilizing the transition state of the reactions.
Work in the field of Molecular Genetics
Pauling, with Harvey Itano, Ibert wells, and Seymour Jonathan Singer, published their work on sickle cell anemia and demonstrating it to be a molecular and genetic disease in 1949. They validated that individuals with this disease contain a modified form of hemoglobin in their blood cells. For this, they used gel electrophoresis to show the difference between normal and diseased hemoglobin proteins. It was also the first demonstration through Mendelian inheritance to understand the physical properties of proteins.
Atomic Nucleus’ Structure
Besides, Pauling presented a Spheron Model to explain the structure of the atomic nucleus. According to this model, we can view the nucleus as a cluster containing different subatomic particles.
John Dalton

John Dalton was a British chemist who lived from 1766 to 1844. Besides being a chemist, he was also a microbiologist and a meteorologist too. He is rightfully known for his work on identifying the heredity of color blindness, his law of partial pressures of gases, and explaining the behaviors of atoms taking into account their weights.
Color Blindness
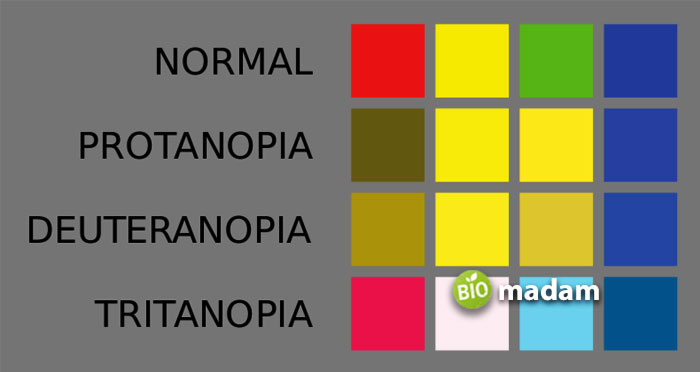
Dalton’s work on color blindness stems from his and his brother’s color blind condition, which provoked him to think this type of disease must be hereditary. His first paper on color blindness was entitled ‘Extraordinary Facts relating To the Vision of Colors.’ Dalton’s theory of color blindness lost faith in his lifetime. Still, later on, the examination of his preserved eyeballs demonstrated that he had a type of color blindness now known as deuteranopia.
Gas Laws
In 1801, Dalton presented his four essays entitled ‘Experimental Essays.’ He worked on the composition of mixed gases, the effects of vapor pressures, the temperature in vacuum and air, and the principles of thermal expansion of gases. In the following years, he came up with laws of partial pressure of gases, also known as Dalton’s laws.
Dalton’s Atomic Theory
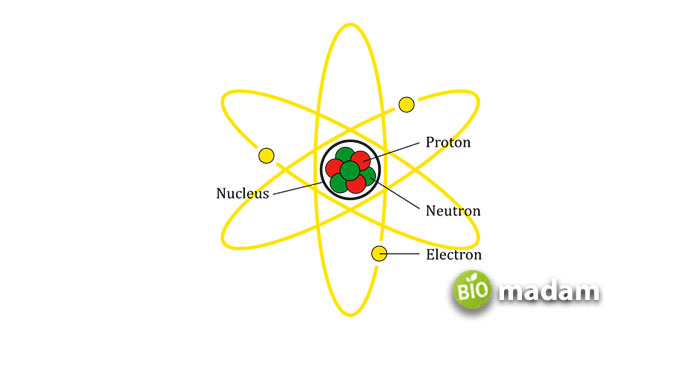
The most important of Dalton’s work was on atomic theory in the field of chemistry. Although his atomic theory remains incompletely understood, some of its inherent postulates are as follows:
- Atoms are the building blocks of all the elements,
- An element is made up of atoms identical in size, shape, and properties, while different elements have different atoms associated with them,
- Atoms cannot be destroyed, created, or subdivided,
- Chemical compounds are formed by the combinations of atoms of different elements, and
- Chemical reactions involve the separation, combination, and rearrangement of atoms.
Dalton’s Atomic Weights
This chemist also presented the concept behind the atomic weights of atoms besides the relative atomic weights. This way, elements can be calculated in relevance to the weight of a hydrogen atom that is conventionally considered one. It assisted Dalton in publishing a table of relative atomic weights of six elements.
Marie Curie

Marie Curie is the first lady to win Nobel prizes, first in physics and then second in chemistry. She is also the first one to win two Nobel prizes together. With her husband Pierre, M. Curie worked on discovering radioactive elements polonium and radium. Later on, she struggled to develop X-rays to account for her most spectacular feats in the world of science.
Discovery of Radioactivity
The discovery of radioactivity by Marie Curie began with the work of another scientist named Henri Becquerel, who was working on the properties of X-rays by using elements that give fluorescence naturally.
At that time, it was believed that when uranium is placed in the sunlight, it gets charged and can be used to develop photographic films (because it emits energy formerly stored from the sunlight).
But the surprise came in Becquerel’s life when he noticed that the energy emitted from uranium indeed developed films without even charging from sunlight. He found them charged upon studying this radiation, negating that they were X-rays (as these rays are neutral).
Marie Curie started investigating this phenomenon further and coined the term radioactivity for it. When the Curies extracted uranium, they found more radioactivity in the leftover than in the pure form. It concludes that the ore contained more than one radioactive element. That is how they discovered polonium and radium from these experiments.
Conclusion
There is a lot of versatility in chemistry, which is the combined efforts of chemists, researchers, and other scientists. Whatever we are studying today or the concepts we apply daily results from the scientists’ efforts made years ago!

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.

