Proteins are important organic compounds in the body contributing to growth and development. They provide support and movement to the body by forming muscle tissues. Proteins are complex molecules made of building blocks called amino acids. Different types of proteins are based on unique structures depending on their formation. Let’s tell you about the different levels of protein structure in detail.
Protein Structure
The basic protein structure comprises multiple units of amino acids linked together by peptide bonds to form a polypeptide chain. Proteins are formed by twisting one or more polypeptide chains into a 3-D shape comprising numerous loops, folds, and curves. The shape and structure of the protein determines its interaction with other molecules. Furthermore, the chemical bonds including ionic, covalent and metallic bonds between molecules in the polypeptide chain play a critical role in shaping proteins. Proteins are categorized into two types according to their shapes.
Globular Proteins
They form by coiling polypeptides to create a spherical shape and are soluble in water. Hemoglobin, myoglobin, and different types of antibodies are globular proteins.
Fibrous Proteins
Alternatively, fibrous proteins are elongated and wire-like in texture. The polypeptide chains in fibrous proteins run parallel to each other. They connect by hydrogen disulfide bonds which are usually insoluble in water.
Connective and epithelial tissues comprise fibrous proteins.
Different Levels Of Protein Structure
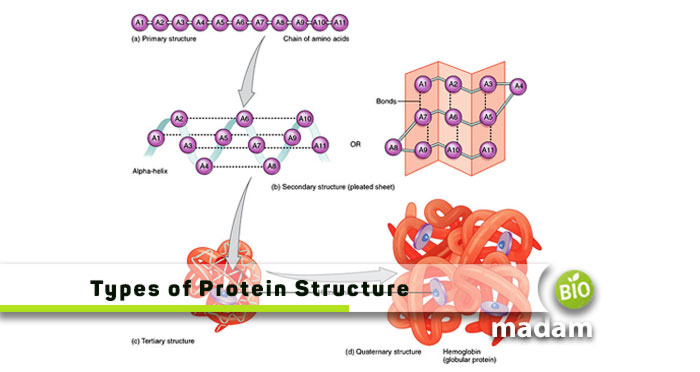
The degree of complexity in the polypeptide chain varies from one protein to another. Some may contain one, while others may have more than one of the amino acid chains. It gives rise to the four protein structures, including primary, secondary, tertiary, and quaternary.
The 3D conformation of these organic molecules relies on the linkages between the amino acids in polypeptide chains. Besides RNA, DNA also plays a significant role in protein synthesis. The shape of the protein and relevant information is already stored in the DNA. Eventually, the new protein may form any of the four types of structures.
- Primary: Linear sequence of amino acids connected by peptide bonds
- Secondary: α-helices and β-sheets formation due to hydrogen bonds between peptide bonds
- Tertiary: Interactions between chains of amino acid arising from the folding of secondary protein structure
- Quaternary: Multi-subunit arrangement of polypeptide chains
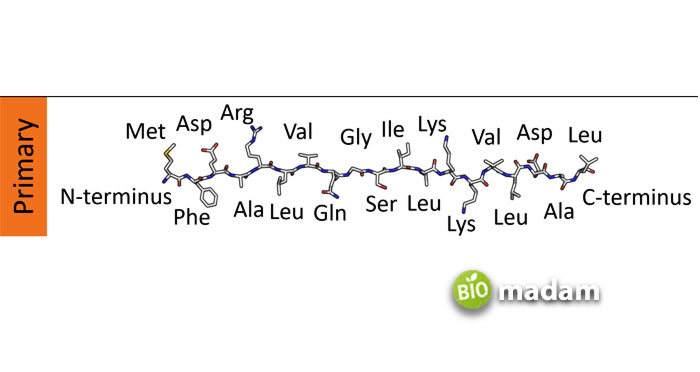
Primary Structure of Proteins
The primary structure of proteins comprises unique amino acids in a linear form. Amino acids join together through peptide bonds to form polypeptide chains. The chains may contain any of the twenty amino acids involved in producing proteins. The order of amino acids in a polypeptide chain determines the characteristics of that particular protein.
Amino acids are formed by a combination of:
- Hydrogen atom
- Carboxyl group
- Amino group
- R (variable) group
All these groups bond to the alpha carbon atom. The R group is particular to each amino acid producing particular protein monomers.
Now, if you wonder how amino acids arrange in a specific manner, the answer lies within the nucleus. The nucleus contains DNA and genes, which contain information about the amino acid sequence. A change or replacement in one amino acid of a protein chain will jeopardize its function.
Genetic disorders often result from mutations causing changes in the primary protein structure. Changes in primary structure also lead to secondary, tertiary, and quarterly structural changes.
For example, changes in hemoglobin may result in sickle cell anemia.
Hemoglobin is a protein comprising two identical α chains and two identical β chains. A single amino acid substitution in normal hemoglobin changes the structure of the protein, resulting in sickle cell anemia. The amino acid Valine replaces Glutamic Acid in the β chain, which causes the polypeptide chain to fold slightly differently. This new shape of protein forms a dysfunctional hemoglobin structure. Ultimately, the deformed red blood cells are unable to transport oxygen properly throughout the body.
Secondary Structure of Proteins
The folding of primary protein structures forms the secondary structure of proteins in particular ways. Proteins fold in energy-efficient shapes known as the native state in varying structural levels. The secondary structure forms in the cytosol or cytoplasm of the rough ER, next to the smooth ER, by promoting linkages between different amino acid chains and water.

The polypeptide chain folds into ribbons and spirals of α-helices and β-sheets with gentle and not-gentle twists. The folds result from the interaction between the carboxyl and amine groups of the peptide bond. The alpha helix contains α-carbons in rotations providing suitable angles to build strong hydrogen bonds. On the other hand, β-sheets have multiple β strands bound together with hydrogen bonding. The polypeptide chains in β-sheets may have parallel or anti-parallel bonds. Parallel sides are typically on the inside of the protein structure, while anti-parallel bonding brings more stability.
Moreover, protein chain segments may also have local folds, which are comparatively simpler. They are usually in the shape of a loop or spiral and are known as secondary elements.
You can study the components and structure of secondary proteins using NMR and X-ray diffraction techniques. X-ray diffraction is different from X-ray and MRIs used generally. It gives a 3-D map of the electrons in the protein, while NMR identifies the spacing between proteins. They help determine the folds and overall structure of different proteins.
α-helix and β-pleated sheet structures play an important structural role in most fibrous and globular proteins.
Tertiary Structure of Proteins
The tertiary structure of protein formation refers to folds that result in a 3-D structure. The secondary structure may combine to form a 3-D or tertiary structure in different ways. The three-dimensional folding of α-helices and β-sheets resulting from noncovalent interactions in the molecule form these protein structures. The interactions occur between polar, non-polar, basic, and acidic R groups in the polypeptide chain. The hydrophobic groups on the amino acid sequences are on the inner side, whereas the hydrophilic groups are on the outside.
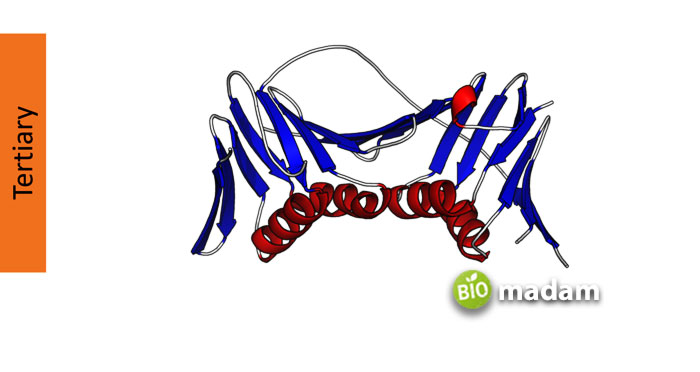
Hydrophobic interactions, disulfide linkages, hydrogen bonds, and van der Waals forces also contribute to tertiary structures in proteins.
Hydrogen Bonds
Hydrogen bonds are formed between the R groups of amino acids and polypeptide chains. These bonds give rise to ionic bonding besides covalent bonds due to protein folding. The ionic bonds result from the interaction of negatively and positively charged R groups.
Disulfide Linkages
Tertiary structures are not as stable and often change their shape during their lifetime. The proteins solidify their structures by disulfide bonds. The disulfide bonds are formed between the R groups of two cysteines.
Hydrophobic Interactions
The R groups contributing to protein structure in any amino acid could be hydrophilic or hydrophobic. Amino acids with hydrophilic R groups interact with their environment, whereas negative R group amino acids move towards the center of the protein and facilitate structure formation.
Van Der Waals
While the role of van der Waal’s forces is not as significant as disulfide or peptide bonds, they help stabilize tertiary protein structure. They are formed between polarized molecules and contribute to holding them together.
Tertiary protein structures contain “functional domains” that determine the protein’s function. The loss of the three-dimensional shape of tertiary proteins will result in the loss of protein function.
Quaternary Protein Structure
Quaternary proteins are made of subunit proteins comprising more than one polypeptide chain into a functional multimeric protein. Proteins with one polypeptide cannot have a quaternary structure. The weak linkages between subunits in multiple sub-unit proteins help stabilize the protein structure. These bonds are typically disulfide and hydrophobic interactions. Various enzymes also play a major role in creating quaternary structures.
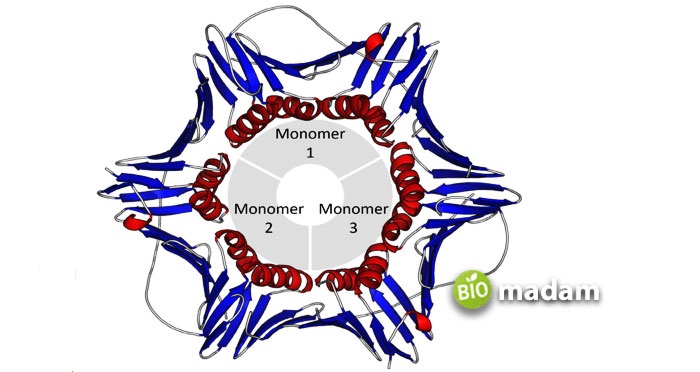
The protein subunits may be of the same or different types. Quaternary proteins made with the same subunits are indicated by ‘homo’ while different ones are called ‘hetero.’
Insulin is a common example of quaternary globular protein containing disulfide and hydrogen bonds binding the polypeptide chains.
Determining the Protein Structure
Any level of protein structure is determined by its primary structure. The primary structure constitutes different amino acids arranged in a specific manner. The genes in a cell carry instructions about protein synthesis. The nucleic acid allows DNA to uncoil and transcribe into RNA through DNA transcription. Further, the specific RNA produces proteins according to the information by translation. Each of these proteins forms a unique structure and performs specific functions in the body.
The Bottom Line
All living organisms around us, including animals, plants, and fungi, comprise proteins. Proteins, carbohydrates, and fats are essential to normal body functioning. Proteins particularly contribute to body mass and growth. Primary, secondary, tertiary, and quaternary proteins represent the different levels of protein structure. Primary protein structure is the basic structure for all other proteins. They comprise twenty amino acids arranged in specific manners for different proteins. The protein structure is determined by the genes and alleles that carries information for the specific protein.
FAQs
What are the 4 levels of protein structure?
The four levels of protein structure are primary, secondary, tertiary, and quaternary. They form different shapes according to the degree of complexity of their polypeptide chain.
What is the difference between tertiary and quaternary structure?
Tertiary structure refers to a further fold in the secondary structure that gives rise to a 3-D formation. On the other hand, quaternary proteins comprise more than one polypeptide chain. Proteins with single polypeptides cannot possess a quaternary structure.
How do you know if carbon is primary, secondary or tertiary?
Carbons attached to one other carbon atom are primary. Those joined to two carbons are secondary, while carbons joined to three more carbons are tertiary.

The Biomadam Content Team develops and manages educational and informational articles published on Biomadam. Content is prepared using standard reference materials and follows internal editorial guidelines to ensure clarity and consistency.