Recently updated on March 4th, 2024 at 12:53 pm
Chemistry is a broader field of science that helps us study the formation and separation of chemical substances through its various branches. You can utilize various techniques to form a new substance, separate two already merged components, or mix the two different phases/substances. Among the many well-known processes is chromatography, which has been assisting the world of chemists since 1900. There are different types of chromatography, including column chromatography.
We have extracted all the primary types of column chromatography in this article, but before that, let us understand the principle of column chromatography.
Principle and Purpose of Column Chromatography
In chemistry, the technique that is used to segregate an individual compound from a complete mixture is called column chromatography. Since a column is used in this separation technique, it is named column chromatography. This process works on the fundamental principle of partitioning the stationary and mobile phases.
The stationary phase here is usually a solid component packed inside a column, such as silica gel or beads, whereas a mobile phase can be a liquid or a gas that flows through the column. Right when the mobile phase travels from the column, different components hit the stationary phase at various degrees, causing elution at variable times. Laboratory experts often employ this biology technique to separate biological compounds such as nucleic acids (DNA or RNA) or proteins from a mixture (also dissolved in a suitable solvent).
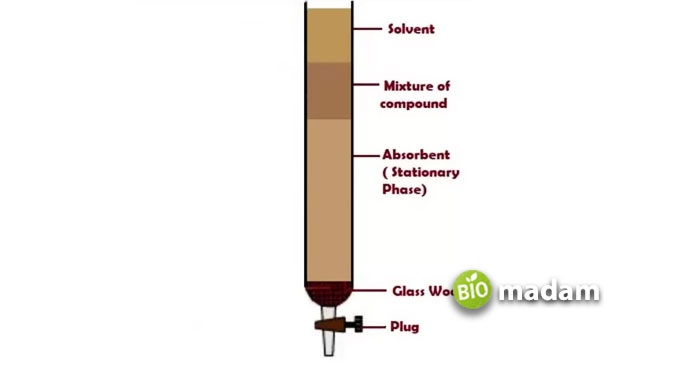
Figure 1. a regular column used in chromatographic separations and their different parts.
Types of Column Chromatography
Column chromatography has helped the chemical industry through its various types, which we will explain below. There are seven main types of column chromatography, such as:
- Adsorption chromatography
- Ion exchange chromatography
- Gel filtration chromatography
- Affinity chromatography
- Gas chromatography
- Partition chromatography
- High-performance liquid chromatography
Let us now briefly examine these different column chromatography types with understandable examples where possible.
Adsorption Chromatography
It is the oldest type of column chromatography, which retains the compounds from the mixture on the mobile phase, adsorbed on the surface of the stationary phase. Usually, the forces involved in binding the compound with the solid support are Van Der Waals forces and steric interactions. Besides, adsorption chromatography is referred to as solid-liquid chromatography. This chromatography was first invented by the Russian botanist Mikhail Tsvet in 1901. Figure 2 illustrates how molecules might become adsorbed on the surface of the solid support.
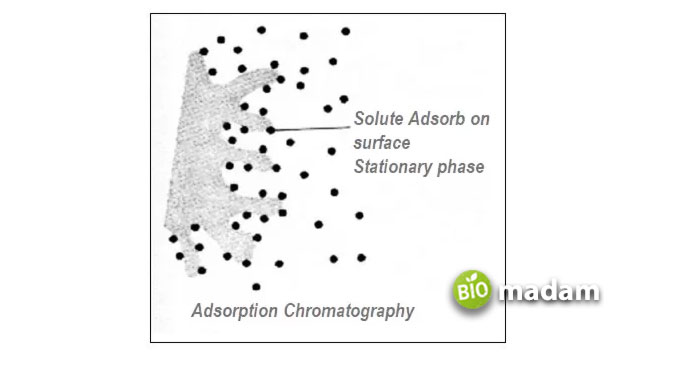
Figure 2. Adsorption of solute molecules on the surface of the adsorbent.
The invention of adsorption chromatography is associated with its very first example. The carotenoid and chlorophyll a and b pigments were formerly separated using calcium carbonate, sucrose, and alumina as a stationary phase, with petroleum and ether/ethanol mixtures as eluents. Adsorption chromatography is further utilized to separate amino acids and fatty acids. Besides, this technique is implied to isolate antibiotics and determine peptides.
Ion Exchange Chromatography
Ion chromatography or ion-exchange chromatography is another type of column chromatography that mostly separates polar and ionic compounds. The basic principle lies in the molecules’ charge that separates by attaching to the oppositely charged stationary phase.
Then, finally, in the elution step, the molecules of the eluent compete for the ionic compound being purified, take their places in the stationary phase, and thus the ionic compound is purified (see Figure 3).
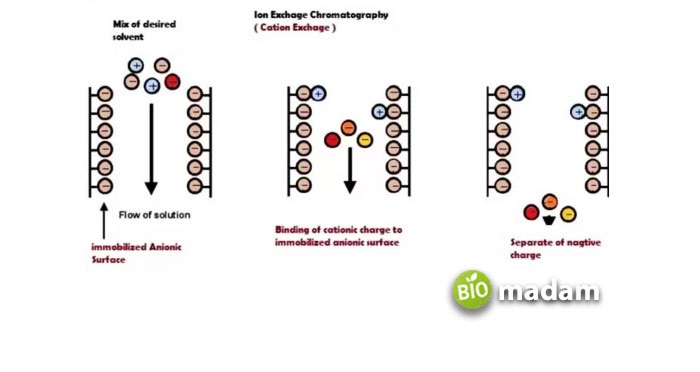
Figure 3. Ion exchange column chromatography. This particular type is anion exchange (negative ions are being exchanged)
For example, let us look at an anionic resin quaternary amine (-N+(CH3)3). It is a strong anion exchanger with a commercial name of Q resin. As it is an anion exchanger (having a positive charge on itself), it helps purify various negatively charged compounds. However, there are some disadvantages observed too.
Gel Filtration Chromatography
Gel filtration chromatography is also known as size exclusion and molecular sieve chromatography. It is a separation technique where molecules in a mixture separate based on their sizes or molecular weights. Its most common applications are in separating macromolecular complexes, such as proteins and polymers of industrial importance.
The resins used for gel filtration chromatography consist of spherical particles or beads that form a porous matrix upon the column’s packing. These beads are non-reactive and non-adsorptive. The molecules larger than the pore size of the column elute first since these are of different resins and particular sizes.
The molecules having sizes following the magnitudes of the pores (or smaller than them) would penetrate them. Furthermore, they will elute so that the smallest molecules will come last since they completely enter the pores (Figure 4).
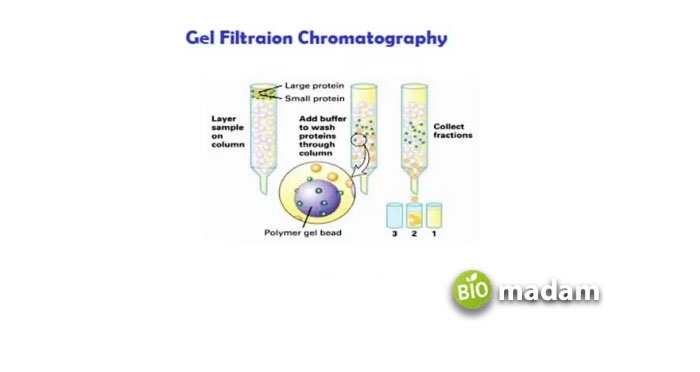
Figure 4. The symbolic representation of the principle of gel filtration chromatography. In red are the molecules of large size which do not enter the resin’s pores and thus elute early. While blue particles, smaller in size, trap inside the resin’s pores and take longer to elute than the larger molecules.
Affinity Chromatography
It is a type of column chromatography where biomolecules disintegrate from a mixture based on their specific interactions with the resin. It utilizes the high specificity and interaction of two biological molecules in its principle.
The purification and separation of the desired compound from the mixture is accomplished when the molecule of interest (called the ligand) interacts with its target attached to the surface of the stationary phase. It is then eluted with the help of a solvent, which competes with it for binding sites on the stationary phase (Figure 5).
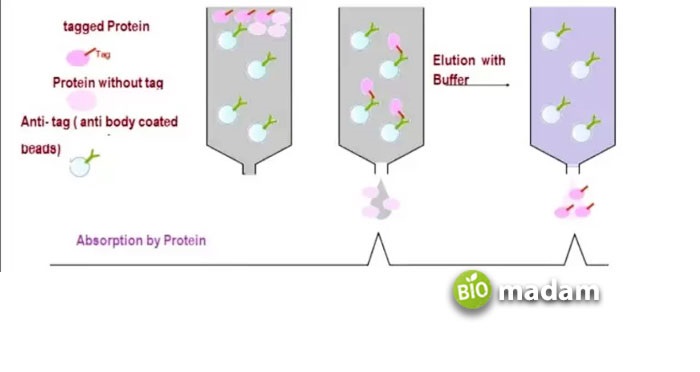
Figure 5. the principle of affinity chromatography.
The most commonly used application of affinity chromatography is separating a protein from a mixture. It is usually processed by using the column or resin containing the antibodies for that protein attached. Therefore, when the protein solution passes through the column, only the protein of interest attaches with its antibodies, and the rest washes out. However, affinity chromatography has some disadvantages, too.
Gas Chromatography
It is another type of chromatography that helps to detect and analyze molecules and compounds in their vapor form.
The mobile phase consists of an inert carrier gas containing the examination sample. The stationary phase is a thin microscopic layer of a polymer or liquid attached to the inside of a column. Experts usually term it vapor-phase chromatography or gas-liquid partition chromatography.
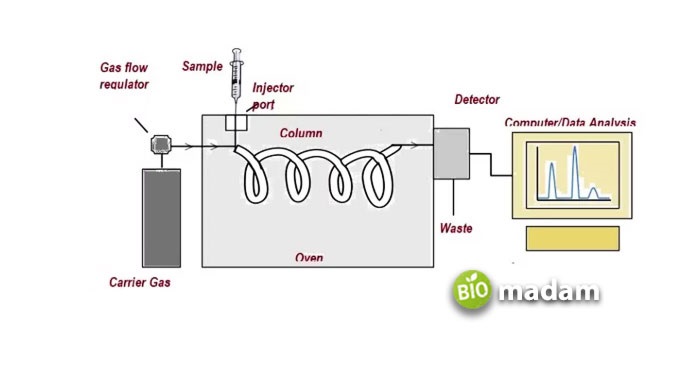
Figure 6. the diagrammatic representation of gas chromatography. The sample in the gaseous form passes through the column, where the components detect and further record in the form of peaks. The data gathered is then analyzed.
Partition Chromatography
Another type of column chromatography mainly separates the mixture components into two liquid phases based on their partitioning behavior. The liquid surface here is immobilized by a stationary phase, whereas the mobile phase moves away – separating the components. Several partition coefficients affect the partition of mixtures in this type of chromatography.
This type of column chromatography has broadly helped detect and separate color mixtures, including pigments. Just like adsorption chromatography, this type of column chromatography also helps determine lipids, proteins, glycosides, and other biomolecules. Moreover, different large-scale pharmaceutical industries utilize partition chromatography to examine and purify drugs.
High-Performance Liquid Chromatography (HPLC)
It is also a separation technique extensively utilized in analytical chemistry and biochemistry. Also known formerly as high-pressure liquid chromatography, it differs from regular liquid column chromatographic techniques in that it involves the application of high pressures (50-35- bars) for its operation.
The basic principles are quite similar to traditional column chromatography. The adsorbent initially attaches to the column walls through which the solvent containing the compound passes with pressure. The attached compound further elutes with an appropriate elution buffer
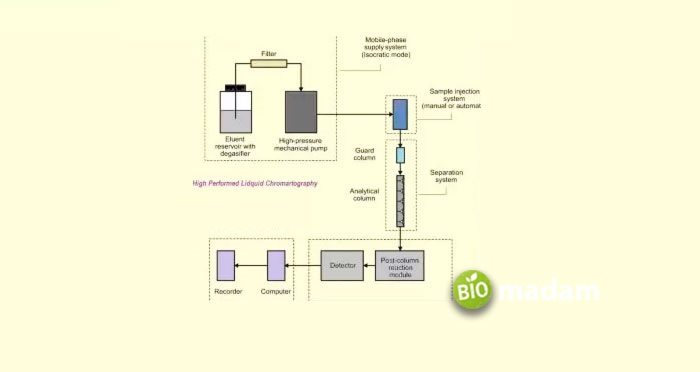
HPLC strengthens a wide variety of fields by applying them. For example, it is broadly used in the manufacturing industry to produce biological and pharmaceutical products. Most of the recombinant proteins commercially available for research purposes are purified using HPLC. Besides, it also helps in conventional and forensic research and other medical purposes.
Why is Column Chromatography Useful?
Column chromatography has proved to be a helpful technique due to its versatility and efficiency. It has the ability to separate complex mixtures that sometimes share the same properties and similar structures. This process has helped the world of biochemical research by providing different stationary and mobile phases.
It plays a crucial role in isolating active ingredients – from small organic compounds to large biomolecules, like proteins, carbohydrates, and nucleic acids. Moreover, as discussed before, column chromatography assists the pharmaceutical industries in determining drug estimation from its main formulations. Laboratory technicians have implied it to remove impurities while performing their lab tests. Overall, many scientists, chemists, and biochemists utilize column chromatography because of its efficiency and cost-effectiveness.
Column Chromatography – A Versatile Approach
In a nutshell, column chromatography offers a wide range of applications in the chemical world. We cannot deny how this type of chromatography has assisted in purifying and separating different phases of matter. It actively plays a role in isolating major constituents and helps disintegrate complex mixtures. Whoever joins the field of chemistry would see column chromatography and its various types doing wonders in biochemical and biotechnological research

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.