Acids and bases are common terms used in chemistry to express different types of chemicals. Acids are typically considered harmful substances and mixtures that can burn your skin. Sulfuric acid is the strongest acid used in research and other applications. On the contrary, bases are not as dangerous, but they must be handled carefully. Acids and bases are also utilized in disinfection and sterilization.
Keep reading to learn all the differences between strong and weak bases.
Comparison Table
| Characteristics | Strong Bases | Weak Bases |
| Ionization | Complete | Partial |
| Proton Removal Ability | Present | Absent |
| pH | 10 – 14 | 7.3 – 10 |
| Equilibrium Constant | High | Low |
| pKb Value | High | Low |
| Reactivity | High | Low |
| Conductivity | High | Low |
What are Bases?
A base is a molecule that takes a proton from an acid. Bases also contribute to neutralization reaction on reacting with acid. Reactions between acids and bases produce water and salt.
Bronsted Lowry’s theory states that a base reacts with the acid and brings up a proton. They can be used as liquids or aqueous solutions in chemistry experiments. Bases are categorized as a strong base, neutral base, and weak base. They vary according to their separation of ions in a solution. A strong base can separate a hydrogen ion from a weak acid. In contrast, a soft base has less ability to separate a hydrogen ion from an acid.
Properties of a Base
- Bases turn red litmus paper to blue
- Bases are bitter in taste
- They feel like slime when touched
- When bases come in contact with acidic substances, they react violently
- Bases have high and low electrical conductivity
- Bases are caustic on organic things
- Alkalis are bases soluble in water
What are Strong Bases?
Strong bases get fully separated in order to provide a solution of ions.
There are strong bases, such as hydroxides of alkali and alkaline earth metals, that can be harmful as they are caustic. Strong bases can remove the transfer of proton of weak acidic C-H groups. This can happen in the absence of water also. The concentration of ions decides the level of electrical conductivity. So, a strong base solution has more ions, which is why they have a high level of electrical conductivity.
Examples of Strong Bases
Examples of strong bases are:
- Cesium Hydroxide (CsOH)
- Sodium Hydroxide (NaOH)
- Potassium Hydroxide (KOH)
- Barium Hydroxide (Ba(OH)2)
- Calcium Hydroxide (Ca(OH)2)
- Lithium Hydroxide (LiOH)
- Strontium Hydroxide (Sr(OH)2)
- Rubidium Hydroxide (ROH)
What are Weak Bases?
Weak bases leave OH– ions (anion) by taking hydrogen ions (cations) from water.
The basicity of an aqueous solution is referred to as pH. Weak bases solutions have a higher hydrogen ion concentration than hydrogen ion of strong bases. Weak bases rarely accept protons, which is why they have high H+ concentrations in the solution. Since bases are proton acceptors, the base receives an OH– ion from water. Weak bases are less completely protonated than stronger bases and, therefore, have a higher H+ concentration in the solution.
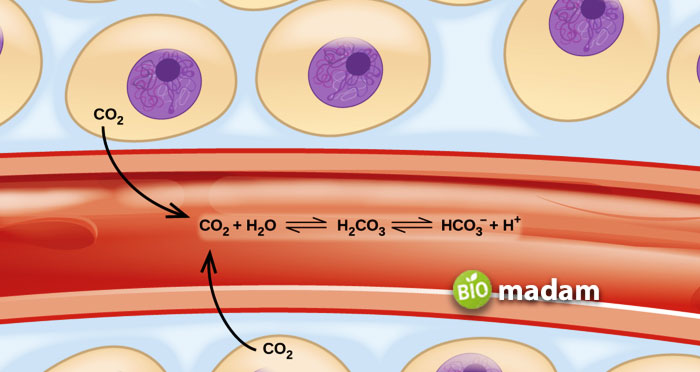
Weak base shifts towards the left side of the equilibrium due to its properties. The weaker base will shift toward the left side, the lower the value of the constant will be. The concentration of ions decides the level of electrical conductivity. Therefore, weak bases solution has fewer ions and low electrical conductivity. Some solutions and suspensions used in manufacturing may contain weak bases.
Examples of Weak Bases
Examples of weak bases are
- Ammonia (NH3)
- Copper Hydroxide (Cu(OH)2)
- Lead Hydroxide (Pb(OH)2)
- Aluminum Hydroxide (Al(OH)3)
- Ferric Hydroxide (Fe(OH)3)
- Aniline (C6H5NH2)
- Trimethylamine (N(CH3)3)
- Zinc Hydroxide (Zn(OH)2)
Difference Between Strong Bases and Weak Bases
Ionization
Strong Bases
A strong base is also known as a base that ionizes completely in an aqueous solution.
Weak Bases
On the other hand, weak bases cannot ionize completely in an aqueous solution. They cannot withdraw H+ ions from acids in an acid-base reaction.
Donation of Hydrogen Ion
Strong Bases
A strong base can remove protons from weak acids.
Weak Bases
In contrast, a weak base cannot remove protons from acids.
pH Level
Strong Bases
The pH level of strong base is from 10 to 14.
Weak Bases
Similarly, the pH level of weak base is from 7.3 to 10.
Level of Equilibrium
Strong Bases
Strong Bases have a high equilibrium constant.
Weak Bases
In contrast, weak bases have a low equilibrium constant.
pKb Value
Strong Bases
Strong bases are known to have a high pKb value.
Weak Bases
Weak bases, on the contrary, are seen to have low pKb value.
Reactivity
Strong Bases
They are highly reactive, for example, concentrated sodium hydroxide can cause severe burns on the skin.
Weak Bases
On the other side, weak bases are less reactive than the strong ones.
Precautions
Strong Bases
Strong bases can damage the skin and eyes. They can be immensely dangerous if ingested or inhaled.
Weak Bases
Weak bases can also be harmful as they may burn skin.
Electrical Conductivity
Strong Bases
Strong bases solution has a high conductivity of electricity. That is why they are good conductors of electricity.
Weak Bases
Weak bases solution has low conductivity of electricity. Therefore, they are poor conductors of electricity.
The Bottom Line
Bases and acids are used in different branches of chemistry to study the nature of substances round us. Bases can be strong, weak, or neutral depending on their ionization in water. Strong bases are good conductors of electricity, while weak bases are considered electric insulators. The pH is a major difference between strong and weak bases. Weak bases have a pH of 7.3 to 10, whereas strong bases range between 10 and 14. Both are important parts of chemical experiments in labs.
FAQs
What is the difference between a strong base and a weak base dissociation?
Strong bases exhibit complete dissociation to produce ions in solutions. On the other hand, weak bases do not dissociate completely to form ions.
Is NaOH strong or weak?
NaOH is a strong base and electrolyte with a pH of 10 that dissociates almost completely in a solution to give OH- ions.
Which is stronger KOH or NaOH?
NaOH is a stronger base compared to KOH. NaOH dissociates faster and more efficiently in solution than KOH.

Hello, I would like to introduce myself to you! I am Chelsea Rogers, an experienced blog writer for science articles, holding an MPhil degree. My enthusiasm to grab the best knowledge, let it relate to botany, zoology, or any other science branch. Read my articles & let me wait for your words s in the comment section.