Immunity is a critical part of human physiology that helps protect our body against diseases. It is a combination of different cells including antibodies, macrophages, killer cells, B cells, and T cells. Different types of antibodies act on particular signals to fight the invaders. They kill pathogens and protect your body from harm. IgG and IgM are two of the five immunoglobulins (antibodies) in our body.
Let’s tell you the differences between IgG and IgM in detail.
Comparison Table
| Characteristics | IgG | IgM |
| Acronym For | Immunoglobulin G | Immunoglobulin M |
| Immune Response Type | Secondary | Primary |
| Percentage | 80 – 85% | 10% |
| Size | Smallest | Largest |
| Mol. Weight | 150kDa | 900k and 1000kDa |
| Location | Blood, lymph, and the intestines | Blood and lymph fluids |
| Distribution | Intra and extracellular regions | Intracellular region |
| Structure | Two small and two heavy chains | Heavy chains of 110, light chains of 220 a.a. |
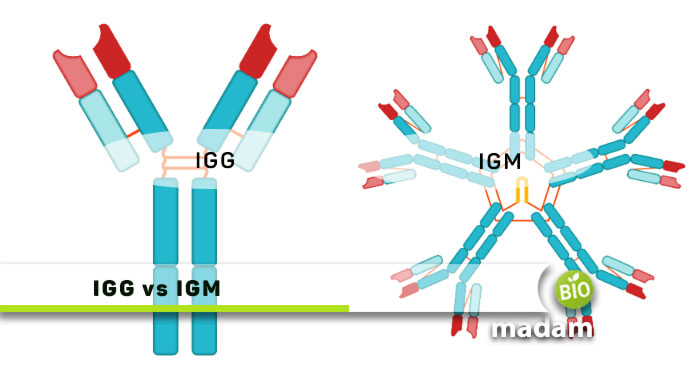
| Monomer Shape | Single Y-shaped unit | 5 Y-shaped units |
| Binding Sites | Two antigen-binding sites | Ten binding sites |
| Mechanisms | Complement fixation, neutralization, & opsonization | Agglutination and cytosolic reactions |
| Carbs | 3% | 12% |
| Symptoms Appearance | 7 to 14 days | Within the first 7 days |
| Half Life | 23 days | 5 days |
| Placental Passage | Yes | No |
What are Immunoglobulins?
Immunoglobulins are antibodies made of globular proteins. They recognize and bind to antigens like bacteria and viruses to destroy them. The function of immunoglobulins is highly specific and complex. There are five types of immunoglobulins: IgG, IgM, IgA, IgD, and IgE. Each antibody is produced by B lymphocyte and has a unique structure, distribution, features, and specificity. Keep reading to learn more about IgG and IgM specifically.
What is IgG?
IgG, or immunoglobulin G, is the most common and abundant antibody in the human body. It makes up around 75-80% of the total antibodies in the blood plasma. It is considered the secondary immune response as they develop in the later stages of the immune response. However, it is the only antibody capable of crossing the placental barrier and providing passive immunity to the fetus. It also travels through the breast milk to the lactating baby.
Immunoglobulin G is widely distributed in the intra and extravascular regions. Their carbohydrate content is lower than other immunoglobulins. IgG immunoglobulins have an immune memory and remember the antigen or pathogen they previously interacted with. These antibodies also have the longest lifespan among all immunoglobulins. Their molecular weight is around 150,000. IgG is further categorized into IgG1, IgG2, IgG3, and IgG4.
Structure of IgG
Immunoglobulin G comprises large globular protein molecules weighing around 150,000 Da. It comprises a quaternary protein structure consisting of two light and two heavy peptide chains. The heavy gamma chains of 50,000 Da are identical, along with two similar 25,000 Da light chains. The resulting structure looks like a Y-shaped antibody.
IgG comprises two basic structural divisions: antigen binding site and constant region. The antigen binding site provides space for the antigen to bind. The FC receptors of neutrophils and macrophages recognize the FC sites.
IgG Functions
These antibodies play a fundamental role in immunity against pathogens by performing functions like complement fixation, neutralization, and opsonization. The most prominent functions of immunoglobulin G are:
- IgG coats the antigens to attract phagocytic cells to kill them.
- Immunoglobulin G induces the complement pathway by attracting complement proteins for binding.
- IgG marks the antigen and helps kill it by poking holes in the plasma and cell membrane.
- Another important function of IgG is the direct neutralization of toxins. IgG binds to the active site of the pathogenic body to prevent bacterial and viral infections.
What is IgM?
IgM or immunoglobulin M, is a component of the body’s first line of defense. It is the primary type of antibody to interact with all types of antigens entering the body. IgM initiates the primary immune response that offers short-term protection from the infection-causing antigen. The concentration of IgM in the body is quite low, accounting for only 5 – 10% of the total. Around 85% of IgM are found in the intravascular compartments. Their life span is around 5 days only. IgM is a potent agglutinin present on the surface of naïve B-cells and red blood cells.
The IgM antibodies may or may not develop as a result of infection. Some IgM antibodies circulate in the blood all the time. However, introducing a disease-causing antigen gives rise to immunoglobulin M antibodies. Thus, they play a significant role in the initial immune response. The concentration of IgM in the body increases due to pathogenic infection and keeps increasing until IgG takes over. Alternatively, natural IgM antibodies in the blood decrease inflammation and prevent autoimmune responses leading to diseases and disorders. They are produced in the fetus at around 20 weeks of conception at early developmental stages.
Structure of IgM
IgM antibodies are way heavier than other types of immunoglobulins and weigh between 90,000 and 1,000,000 Da. They comprise four peptide units forming a pentameric structure. Each of the five monomers has a heavy and light chain. The heavy chain includes around 576 amino acids. They are divided into Constant (CL) and Variable (VL) domains. The structure of IgM constitutes four regions of 110 amino acids, each followed by a tailpiece of 20 amino acids. The light chain has 220 amino acids with CL and VL domains. The shorter chains combine with the heavy chains due to disulfide bridging.
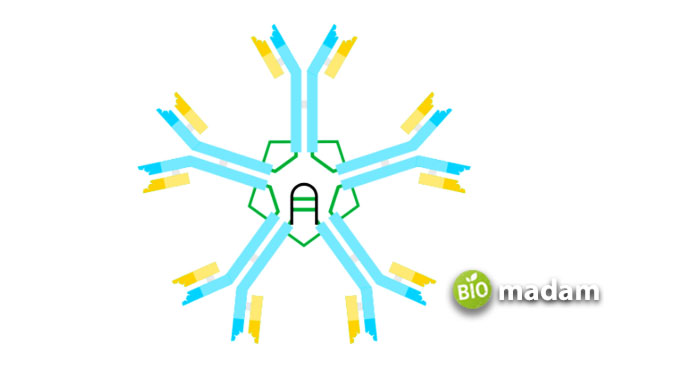
IgM Functions
- IgM antibodies help prevent and detect pathogen-related diseases and infections in the body.
- They produce the primary immune response on exposure to an antigen.
- They initiate the complement system by binding with the C1 component.
- IgM immunoglobulins attack the pathogen until IgG takes over.
- IgM antibodies have their role in the detection of autoimmune diseases like psoriatic and rheumatoid arthritis.
Similarities between IgG and IgM
- IgG and IgM are the antibodies produced in all types of immunity.
- They belong to the adaptive immune system.
- They help in the prevention and detection of infections in the body.
Differences between IgG and IgM
Definition
IgG
IgG or immunoglobulin G is the most abundant antibody in the body producing a secondary immune response.
IgM
IgM or immunoglobulin M plays a significant role in autoimmune diseases and produces the primary immune response.
Size
IgG
IgG is considered the smallest of all five types of immunoglobulins.
IgM
Contrarily, IgM antibodies are the largest immunoglobulins in size.
Presence
IgG
IgG antibodies make up 80 – 85% of the total antibodies in the body.
IgM
While IgM accounts for 10% of the total antibodies in the blood plasma.
Location
IgG
IgG is vastly found in blood, lymph, small, and large intestines.
IgM
Conversely, IgM is produced in the plasma cells and distributed in blood and lymph fluids.
Distribution
IgG
IgG are almost evenly distributed in the intra and extracellular regions, comprising around 45% in intracellular spaces.
IgM
But, a significant proportion (around 80%) IgM is present in the intracellular region.
Molecular Weight
IgG
The molecular weight of IgG is around 150,000 Da.
IgM
On the other hand, IgM antibodies weigh between 900,000 and 1,000,000 Da.
Structure
IgG
Immunoglobulin G comprises two small chains of 25kDa and two heavy chains of 50kDa.
IgM
Whereas immunoglobulin M has four chains of 110 amino acids, each in heavy chains, while the light chains have 220 amino acids in total.
Monomers and Antigen Binding Sites
IgG
IgG consists of a single Y-shaped monomer unit with only two antigen-binding sites.
IgM
On the contrary, IgM is a pentamer comprising 5 Y-shaped units with ten binding sites.
Carbohydrate Percentage
IgG
IgG molecules have a low amount of carbohydrates, accounting for only 3%.
IgM
However, IgM antibodies contain around 12% carbohydrates.
Function
IgG
IgG plays a fundamental role in body immunity by performing functions like complement fixation, neutralization, and opsonization.
IgM
At the same time, IgM antibodies are involved in agglutination and cytosolic reactions.
Role in Immune Response
IgG
IgG produces a secondary immune response, which shows 7 to 14 days post-infection.
IgM
But, the body produces IgM within the first 7 days of infection to fight the pathogen.
Half-Life
IgG
The half-life of IgG is higher than many other antibodies, spanning over 23 days.
IgM
Comparatively, IgM molecules have a short life span of only 5 days.
Placental Passage
IgG
IgG is the only antibody capable of passing the placental barrier.
IgM
Therefore, IgM cannot travel to the fetus by crossing the placental barrier.
The Bottom Line
IgG and IgM are two of the five immunoglobulins and belong to the adaptive immune system. Immunoglobulins are a critical part of human physiology that work together to fight harmful antigens. Structural uniqueness is one of the most significant differences between IgG and IgM. IgG offers two antigen binding sites, whereas IgM possesses 10 binding sites. IgG is the most abundant in the body and delivers a secondary immune response. Contrarily, IgM acts immediately and produces a primary response until IgG takes over. IgM is the largest antibody, while IgM is the smallest.
FAQs
What comes first IgG or IgM?
Studies show that IgM produces the initial immune response within 4 to 7 days of infection. However, the production of IgG may take between 7 and 14 days. These antibodies may be detected weeks, months, or even years later.
Why does IgM switch to IgG?
IgM and IgD often switch to other immunoglobulins like IgA, IgG, and IgE. It allows the antibodies to produce a stronger immune response and kill the pathogens.
What does it mean if I have IgG antibodies but not IgM?
The detection of IgG shows that you have passed the initial infection stage and the pathogen entered the body at least 7 days before. The presence of both IgG and IgM shows that you are in the early recovery phase.

Anna has completed her degree in Pharmacy from the University of Hawaii. She is serving as a research assistant in a pharmaceutical company. She had a great interest in writing blogs, traveling to different parts of the US, and trying delicious recipes in her spare time.