Compounds and mixtures are the basic terminologies used in concepts of chemistry that also play a crucial role in living bodies. Today we will differentiate in detail between compounds and mixtures for your better understanding. Different atoms, when chemically combined, make a compound. On the other hand, a mixture is a blend of two or more substances physically bonded together. These substances can be anything, such as atoms, molecules, and compounds.
Let’s head towards the key differences between the two in tabular form below:
Comparison Table
| Basis of Comparison | Compounds | Mixture |
|---|---|---|
| Brief Definition | Formed by chemically mixing two or more substances | Formed by physically mixing two or more substances |
| Variability in Ratio | Definite Ratio by mass | No fixed mass ratio |
| Nature of Separation | Needs chemical processes for separation | It can be separated through physical methods |
| Role of Constituent Elements | Do not retain their properties | Retains constituent element properties |
| B.P & M.P | Constant | Variable |
| Heat Exchange During Formation | Present | Absent |
| Representation | Through a Chemical Formula | No Formula Representation |
| Nature of Substance | Homogenous | Homogenous or Heterogeneous |
| Substance Type | Pure | Impure |
| Examples | Water, sulfuric acid, nitric oxide, carbon dioxide | Smog, sand & water, & milk of magnesia |
What is a Compound?
Compounds are atomic substances or other elements united together through a chemical bond. This bond can either be ionic, covalent, or metallic, based on the type of compound. All compounds have a fixed ratio of elements; thus, compounds are homogenous. These substances have more distinctive properties than elements that usually combine to present a single compound unit. Moreover, a compound chemically bonded can never be separated by physical means. As discussed, there are several compounds’ types based on their bonding, so we’ll briefly discuss them one by one.
Types of Compounds
Based on Its Bonding
Ionic Compounds
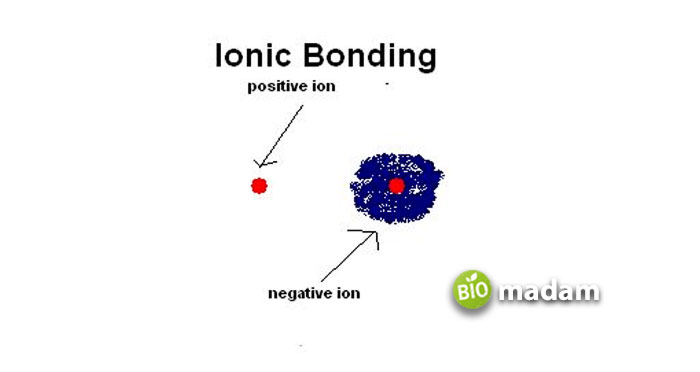
These are also termed salt compounds that are held together through ionic bonds. All ionic compounds form a crystal lattice where many opposite charges surround an ion. Their chemical formula is represented as formula units, for example, NaCl, CuSO4, etc.
Covalent Compounds
Another popular name for covalent compounds is molecular compounds, where all atoms bond together through covalent linkage. Just as the name, the chemical formula of covalent compounds is also called molecular formula, for example, H2SO4, HCl, etc.
Metallic Compounds
Any compound that chemically bonds one or more metal elements together is called a metallic compound. These are good conductors of heat and electricity, for example, NaCl, CaCO3, etc.
Based on the Carbon Presence
Compounds can also be classified depending on the presence of carbon in the molecular structures, such as:
Organic Compounds
All those compounds where one or more carbon atoms are covalently linked to other elements, including hydrogen, oxygen, nitrogen, etc., are called Organic Compounds. Such compounds make up the bulk of a living organism, for example, carbohydrates, proteins, lipids, and nucleic acids.
Inorganic Compounds
According to chemistry, inorganic compounds are all those compounds that lack a carbon-hydrogen direct bond and instead consist of other elements chemically bonded. Some common examples are carbon dioxide, carbon monoxide, ammonia, water, etc.
What is a Mixture?
A mixture is a substance composed of two or more elements physically combined to retain the actual properties of those elements. In other words, a mixture’s properties entirely depend on whatever components are included. We can classify the mixture into three major types depending on its particle size and uniformity.
Types of Mixture
Based on Particle Size

We have solutions, suspensions, and colloids as mixtures for particle sizes.
Solutions
A solution is the homogeneous mixture of two or more components with their properties evenly distributed. These mixtures have small particles that get dissolved, such as a mix of salt and water.
Suspensions
It is a heterogeneous mixture of two or more substances where the large solid particles (greater than 1000 nm) are spread throughout the liquid without dissolving. Furthermore, we can see the suspension particles with naked eyes, for instance, milk of magnesia, muddy water, etc.
Colloids
Colloids are the mixtures of medium-sized particles that remain dispersed and never settle to the bottom of the container, for example, smoke suds, mist, etc.
Based on Uniformity

We can further classify mixtures as homogenous and heterogeneous based on their uniformity.
Homogenous Mixtures
Mixtures having uniform composition and properties throughout their mass and body are called homogenous mixtures. These substances do not show the light passing through them because particle sizes are less than nanometers. All homogenous mixtures have particles in the tiniest sizes that get challenging to differentiate, for example, alloy, the mixture of sand and salt, etc.
Heterogeneous Mixtures
These mixtures lack uniform composition and properties throughout their masses. The boundaries of particles can be easily identified because of having two or more distinct phases. Moreover, these mixtures show the Tyndall effect, for example, a mixture of sugar and water, smog, etc.
Differences between Compounds and Mixture
Both of the substances are entirely different from each other, hence, representing variations discussed below:
Definition
Compounds
A compound contains atoms of different elements chemically combined.
Mixture
On the other hand, a mixture combines two or more substances prepared through physical mixing.
Composition
Compounds
Elements in a compound always have a fixed ratio in a definite manner.
Mixture
On the contrary, a mixture has different components or variable elements, so there is no fixed ratio.
Representation
Compounds
Compounds are generally represented through their formulae representing the number of elements and their symbols.
Mixture
On the other side, the mixture does not have any chemical formula for representation.
Properties
Compounds
The properties of compounds are unique to themselves and utterly different from the properties of their components.
Mixtures
In contrast, the constituents of a mixture do not lose their properties, so a mixture’s properties depend on the type and quantity of substance.
Mass Ratio
Compounds
All compounds have specific mass ratios due to their fixed formulae.
Mixtures
On the other hand, mixtures have variable mass ratios, depending on the ingredients.
Ability to Break Down
Compounds
A compound can only be separated into simpler substances by chemical or electrochemical reactions.
Mixtures
A mixture can be separated into simpler substances quite easily than compounds because of the physical mixing.
Nature
Compounds
All compounds are homogenous due to the fixed ratio of elements.
Mixtures
On the contrary, mixtures are naturally heterogeneous.
Melting and Boiling Points
Compounds
The melting and boiling points of a compound are always defined or fixed.
Mixtures
The melting and boiling points of a mixture are not fixed.
Types
Compounds
Compounds can be classified as covalent, ionic, or metallic substances based on their bonding. Moreover, these are also the organic or inorganic compounds defined w.r.t. presence or absence of carbon-hydrogen bond.
Mixtures
Mixtures are mainly of two types that are homogenous and heterogeneous mixtures.
Heat Change
Compounds
Energy is released or used during the formation of compounds.
Mixtures
On the contrary side, there is no energy involved in the formation of the mixture.
Formation of New Substances
Compounds
A compound is always formed as a new substance with distinct properties whenever two or more elements chemically link together.
Mixture
A mixture is not a newly formed substance; instead, it always retains the properties of its constituent elements.
Examples
Compounds
We have numerous compounds, including water (H2O), Sodium Chloride (NaCl), baking soda, CO2, etc.
Mixtures
Similarly, we have several standard mixtures, such as dental amalgam, vapors in air, colloids such as mayonnaise, milk, salt in water, oil and water, smog, etc.
Summary
The compounds, substances, and mixtures hold great significance in our daily lives. So, neglecting anyone’s properties can be destructive to our bodies. We hope to clear the prominent differences between the two. Compounds and mixtures mainly differ in one being a chemically bonded substance, while the other shows the physical linkage of different atoms.

Anna has completed her degree in Pharmacy from the University of Hawaii. She is serving as a research assistant in a pharmaceutical company. She had a great interest in writing blogs, traveling to different parts of the US, and trying delicious recipes in her spare time.

