All Enzymes are catalysts, but not all catalysts are enzymes.
Enzymes and catalysts are both involved in speeding up or speeding down chemical reactions within and outside the body. They influence the speed of the reaction without being consumed themselves. Catalysts are either organic (different enzymes) or inorganic (non-enzymatic catalysts) in nature.
Comparison Table
| Characteristics | Enzymes | Catalysts |
| Types | Activation and Inhibitory | Organic/Inorganic, & Positive/ Negative |
| Nature | Complex organic molecules | Simple inorganic compounds |
| Composition | Globular proteins | Small molecules, mineral ions |
| Effect on Reaction | Speed up reactions | Fasten or slows down reactions |
| Size | Larger than substrate | Similar to substrate |
| Molecular Weight | High | Low |
| Type of Reaction | Biochemical | Physical |
| Poisonous Protein Effect | Negative effect | No effect |
| Examples | Lipase, Peptidase | V2O5, Platinum |
What are Enzymes?
Enzymes are organic catalysts that increase the speed of reaction. Unlike some other catalysts, enzymes do not decrease the reaction speed.
Around 4,000 enzymes and their activities are known so far. Enzymes are proteins produced by living organisms and are composed of 62 to 2,500 amino acids. Enzymes are substrate-specific and catalyze reactions only that are specific to their need. A very small amount of enzyme is needed to catalyze a reaction. They react in mild temperature and pH conditions. Some enzymes also need cofactors or co-enzymes to assist the action. Cofactors are inorganic ions like Mg2+, Zn2+, Fe2+, and Mn2+, whereas co-enzymes are organic molecules.
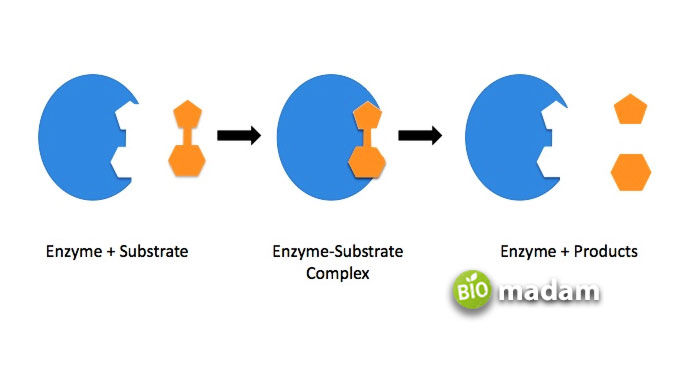
The structure of an enzyme was thought to be like a key that fits in the substrate. The “Lock and Key theory” proposed in 1894 suggested that the substrate is like a lock and the enzyme fits the substrate that it is supposed to go into – just like a key. After believing in this theory for years, another model called the “Induced fir model” was presented in 1958. This model gives the idea that as the enzymes are protein in nature, they flex and alter their shape a little to fit into the substrate. The site where the enzyme fits the substrate is called the Active site. Enzymes have not only active sites for substrates but also cofactors and others.
What are Catalysts?
Catalysts are organic or inorganic compounds that increase or decrease the speed of chemical reactions without being consumed during the process. All biochemical reactions in the body are dependent on catalysts.
The process was first discovered by Gottlieb Sigismund Constantin Kirchhof in 1812 when he observed the breakdown of starch in the presence of sulphuric acid while the acid remained the same at the end of the reaction. Furthermore, the Swedish chemist Jöns Jakob Berzelius named the process “catalysis” in 1835. The name comes from the Greek words, “Kata,” meaning down, and “lyein,” meaning loose.
The discoveries regarding catalysis did not end here, and Louis Pasteur discovered enzymes in sugar fermentation which were named by German physiologist Wilhelm Kühne in 1878. Furthermore, various other catalytic processes and enzymes were discovered. Scientists detected that the catalysts are required in small amounts to alter reaction speed. The catalysts elevate or reduce the reaction speed to act as a temporary intermediate product.
As we mentioned earlier, all enzymes are catalysts, but all catalysts are not enzymes. Catalysts are of two types; inorganic and organic. Organic catalysts (enzymes) are discussed below. Let’s tell you about inorganic catalysts.

Inorganic catalysts are transition metals, like silver, or transition metal oxides. They are typically used to provide a bigger surface area. The increased surface area provides space for the reactions to occur in varying routes, lowering the activation energy. Inorganic catalysts are categorized as heterogeneous or homogeneous catalysts based on the nature of the substance used.
Similarities between Enzymes and Catalysts
- Enzymes and catalysts are typically proteins.
- They decrease a reaction’s activation energy by providing alternate pathways to the reaction.
Difference between Enzyme and Catalyst
Types
Enzymes
Enzymes are usually classified as activation or inhibitory enzymes depending on their function in the body.
Catalysts
They are either organic (enzymes) or inorganic catalysts involved in speeding up chemical and biochemical processes. They are also categorized as positive and negative catalysts.
Nature of Molecule
Enzymes
Enzymes are relatively complex organic molecules.
Catalysts
On the contrary, catalysts are simple, uncomplicated inorganic compounds.
Composition
Enzymes
Enzymes are globular proteins formed by complex structures.
Catalysts
Catalysts are either small molecules, complex compounds, or mineral ions.
Synthesis
Enzymes
Enzymes are produced by ribosomes within the body to facilitate biochemical reactions.
Catalysts
Catalysts are inorganic and thus not synthesized by living organisms.
Effect on Reaction
Enzymes
Enzymes typically speed up the reaction by acting as an intermediate and can be obtained in the primary form at the end of the reaction.
Catalysts
Catalysts work both ways on a reaction. Sometimes they are involved in increasing the speed of the reaction, while sometimes, they decrease the reaction speed without being consumed during the process.
Rate of Reaction
Enzymes
Enzymes speed up biochemical reactions by several folds.
Catalysts
They usually slow down the processes.
Size
Enzymes
Enzymes are large protein molecules and bigger in size than substrate molecules.
Catalysts
Catalysts are usually similar in size to their substrate.
Molecular Weight
Enzymes
Enzymes are organic compounds and thus have higher molecular weight.
Catalysts
Catalysts being inorganic compounds, possess low molecular weight.
Type of Reaction
Enzymes
Enzymes work on biochemical reactions and are a vital part of all reactions within the body.
Catalysts
Catalysts usually act on physical reactions.
Temperature and pH
Enzymes
Enzymes act in a narrow temperature range, and varying temperature and pH affect their activity. They get denatured at high temperatures and are inactive at low temperatures.
Catalysts
Catalysts can perform at a wide variety of temperature ranges and are not sensitive to minor temperature or pH variations.
Effect of Protein Poisons
Enzymes
Poison proteins can alter the function of enzymes.
Catalysts
They are not affected by inorganic catalysts.
Examples
Enzymes
Examples of enzymes include lipase, peptidase, etc.
Catalysts
Vanadium oxide is an example of a catalyst.
The Bottom Line
Catalysts and enzymes are an integral part of many chemical processes inside and outside the body. Enzymes are organic catalysts that facilitate biochemical reactions through different enzymatic regulations. They are thought to modify their shape slightly for the substrate to attach to the active site for necessary action. Common enzymes present in the body include lipase, DNA polymerase, etc., while metals and metal oxides typically act as catalysts.

Anna has completed her degree in Pharmacy from the University of Hawaii. She is serving as a research assistant in a pharmaceutical company. She had a great interest in writing blogs, traveling to different parts of the US, and trying delicious recipes in her spare time.