Recently updated on August 16th, 2023 at 10:03 am
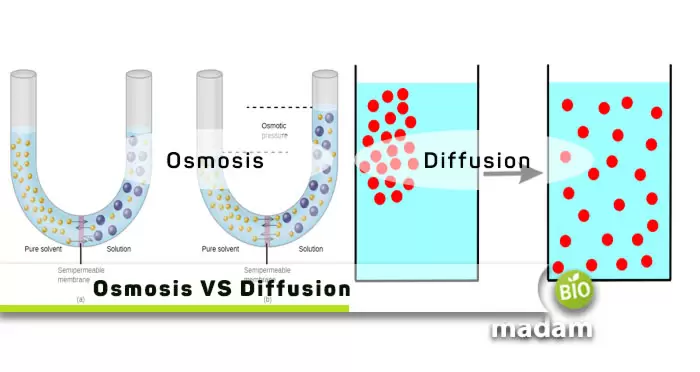
Osmosis, effusion, and diffusion are important natural phenomena that explain various biological activities. These processes take place in organisms within a community, together making an ecosystem. For example, craving water when you eat high-sodium food indicates osmosis. At the same time, the deoxygenated blood in the body receives oxygen through diffusion.
There are multiple differences between osmosis and diffusion, including their principle, semi-permeability of the membrane, and other dependent factors.
Keep reading to understand all the dissimilarities in detail.
Comparison Table
| Characteristics | Osmosis | Diffusion |
| Types | Exosmosis, endosmosis, forward osmosis | Simple diffusion, and facilitated diffusion |
| Medium | Aqueous | All states of matter |
| Semi-permeable Membrane | Present | May/may not be present |
| Molecules | Solvent molecules | Solute or solvent molecules |
| Direction of Flow | Unidirectional | Non-directional |
| Pressure Effect | Reversal | No effect |
| Equilibrium | Not complete | Complete |
| Example | O2 and CO2 exchange | Waste filtration in kidneys |
What is Osmosis?
Osmosis is the process of movement of water or aqueous liquid through a semipermeable membrane from an area of low solute concentration to a higher solute concentration.
Osmosis can be simply explained as placing a solution with a high solute concentration on one side of a semipermeable membrane and a solution with a low solute concentration on the other. The solvent from the low-concentration region passes the semi-permeable membrane to reach the other side.
The movement of solvent from low-solute areas to high-solute regions leads to equilibrium.
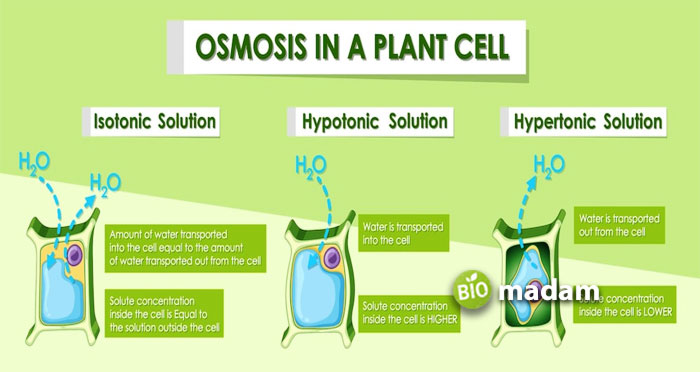
Types of Osmosis
Osmosis takes place due to differences in solute concentration across a semipermeable membrane. It may also include cells surrounded by hypertonic or hypotonic solutions. The classification of osmosis is characterized by the type of solutions involved. The concentration gradients divide osmosis into three types:
Forward Osmosis
Forward osmosis involves the movement of water across a semipermeable membrane to separate dissolved solutes. Forward osmosis uses natural osmotic pressure to carry water to the other side of the membrane in a homogeneous or heterogeneous mixture while retaining the dissolved solutes on the other side.
Endosmosis
Endosmosis refers to the movement of an aqueous medium when a cell is placed in a hypotonic solution. The hypotonic solution contains a lower concentration of solutes compared to the cell. Thus, the water moves to the higher solute concentration, i.e., the cell. Eventually, the cell begins to swell.
Exosmosis
Exosmosis is the movement of water from the cell into a hypertonic solution. The water moves out of the cell as the solute concentration in the surrounding solution is higher than in the cell. As a result, the cell loses its water content and shrinks (cell plasmolysis).
Examples of Osmosis
- Craving for water after a meal with high salt content
- Chemiosmosis during photosynthesis and respiration
- Movement of cellular waste across the cell membrane of plant and animal cells.
What is Diffusion?
Diffusion refers to the movement of compounds and molecules along the concentration gradient. The particles move from a region of higher concentration to a lower concentration.
While osmosis is often considered a type of diffusion, other types of diffusion do not require a semipermeable membrane. Diffusion facilitates the movement of solids, liquids, and gasses in and out of the cells.
The movement of molecules along the concentration gradient helps achieve equilibrium.
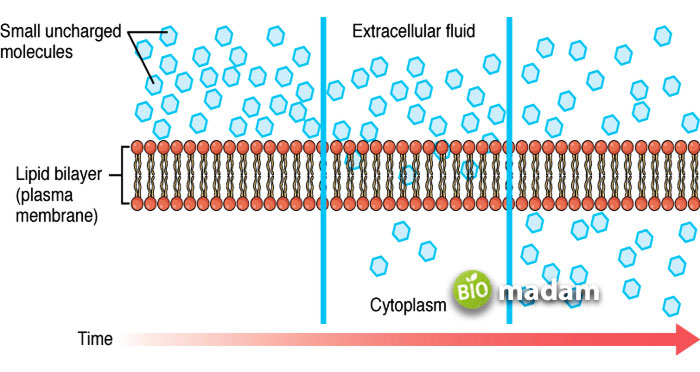
Types of Diffusion
The types of diffusion are classified according to the mechanism of diffusing particles from one area to another. Usually, diffusion occurs without the need for energy. Yet, some processes may require the help of membrane proteins to carry out diffusion. Diffusion is classified as simple and facilitated diffusion.
Simple Diffusion
Simple diffusion is the most common type of diffusion that takes place without any external factors. The particles move along the concentration gradient and thus do not require energy.
Facilitated Diffusion
As the name indicates, facilitated diffusion refers to the movement of molecules from one area to another through the help of other molecules. Membrane proteins in the cell membrane layer help transport particles across concentrations. The proteins allow only specific molecules to pass through the membrane restricting the rest.
Examples of Diffusion
- Calcium diffuses into the blood when signaled by the thyroid gland, a type of endocrine gland.
- Glucose diffusion into body tissues after the absorption
- The deoxygenated blood in the body receives oxygen through diffusion
Similarities Between Osmosis and Diffusion
- Osmosis and diffusion involve the movement of particles from one place to another.
- They work to achieve equilibrium in a particular situation.
- Both processes primarily exhibit passive transport and do not require external energy to push particles.
Differences Between Osmosis and Diffusion
Definition
Osmosis
Osmosis is the movement of aqueous molecules from a solution of lower solute concentration to a higher concentration through a semipermeable membrane.
Diffusion
Diffusion is defined as the transport of particles from a higher concentration to a lower concentration to achieve equilibrium.
Types
Osmosis
The three basic types of osmosis are endosmosis, exosmosis, and forward osmosis. They depend on the concentration of solution around the cell.
Diffusion
Diffusion is categorized into two fundamental types according to the movement mechanism. Simple diffusion does not require other molecules, while facilitated diffusion uses membrane proteins.
Medium
Osmosis
Osmosis occurs only in a liquid medium in the presence of aqueous molecules.
Diffusion
Oppositely, diffusion is not dependent on any one state of matter and may take place in solids, liquids, or gasses.
Membrane
Osmosis
Osmosis requires a semi-permeable membrane that does not allow movement of the solute particles.
Diffusion
On the contrary, diffusion may take place without the presence of a semi-permeable membrane.
Molecules Involved
Osmosis
Only solvent molecules move from a low concentrated solution to a highly concentrated solution.
Diffusion
Whereas solute or solvent particles may move from higher concentration to lower concentration in diffusion.
Direction of Flow
Osmosis
The particle flow is unidirectional due to concentration difference and the presence of the membrane.
Diffusion
Alternatively, diffusion may occur in any direction across a medium without energy.
Pressure Effect
Osmosis
You can slow down, stop or reverse osmosis by applying hydrostatic and turgor pressure.
Diffusion
On the other hand, diffusion is an independent process that cannot be stopped or reversed due to additional pressure.
Example
Diffusion
The exchange of carbon dioxide and oxygen dissolved in water takes place through diffusion.
Osmosis
The filtration of water and waste materials in the kidney occurs through osmosis.
The Bottom Line
Osmosis and diffusion are important biological and physical processes in our daily lives. The most significant difference between osmosis and diffusion is the movement principle. The solvent moves against the concentration gradient in osmosis. Contrarily, particles in diffusion travel along the concentration gradient from higher to lower concentration. While osmosis is restricted to aqueous solutions, diffusion may occur in solids, liquids, and gasses alike.
FAQs
What is the main difference between osmosis and diffusion?
The main difference between osmosis and diffusion is the restriction of osmosis to water molecules, whereas diffusion involves all types of particles.
What are the 3 types of osmosis?
The three types of osmosis include endosmosis, exosmosis, and forward osmosis depending if the solution is hypertonic, hypotonic, or isotonic.
How are osmosis and diffusion related?
Osmosis is considered a type of diffusion in which the movement of water molecules takes place across a semipermeable membrane.

Hello, I would like to introduce myself to you! I am Chelsea Rogers, an experienced blog writer for science articles, holding an MPhil degree. My enthusiasm to grab the best knowledge, let it relate to botany, zoology, or any other science branch. Read my articles & let me wait for your words s in the comment section.