People had a misconception in the previous times where they thought that the molecules and compounds are coming from animals and plants are organic. In comparison, those from a non-living source or mineral extractions are inorganic compounds. However, the latest explanation of organic compounds varies from this! Today we define such compounds as:
Any substance with hydrocarbons (carbon and hydrogen) or its derivatives is called an organic compound.
A branch of chemistry dealing with the extensive study of these organic compounds, including their structure, characteristics, and chemical reactions, is called organic chemistry. This discipline constitutes a large class of chemical compounds linked to carbon atoms as essential components. It has a few exceptions, such as carbides, cyanides, and carbonates.
We copiously use organic compounds in our daily life, so they hold great importance. We will look in detail at how these compounds exist in their various forms and are essential to our living regimes. But before that, let’s comprehend the characteristics of these compounds.
Primary Characteristics of Organic Compounds
We should have a detailed knowledge of the properties of organic compounds for our daily use. The important ones are:
- The carbon atoms in an organic substance are covalently bonded to each other to provide stability
- These comparatively have lower melting and boiling points than the inorganic compounds.
- Similar to carbon-carbon bonds (single or double), the carbon-hydrogen bond also shows non-polarity.
Organic Compounds of Daily Life
We don’t even know and still are using organic compounds in our everyday life. Nature constitutes numerous sub-types of organic compounds, with the fundamentals as nucleic acids, carbohydrates, fats, proteins, etc. Let’s discuss some of its examples below!
Sucrose
It is a disaccharide carbohydrate, a chemical name for ordinary table sugar (white sugar). Other traditional forms of sucrose include brown sugar, powdered sugar, and honey. We usually use sucrose as a sweetener in several foods and beverages, including soft drinks, juices, cakes, and bakery products. Besides its refined usage, sucrose is also available in various fruits and vegetables. Many health associates recommend limiting sucrose intake to six and nine grams for men and women, respectively, as an excess of everything is bad.
The advancement in research helped prepare artificial sweeteners of sucrose for patients with diabetes. Hence, even a diabetic can intake artificial sucrose in sucralose, aspartame, and saccharin. Furthermore, it serves as the body’s first and direct energy source by yielding two glucose molecules through metabolism. Besides, a living body stores excessive sucrose as glycogen (a significant energy reserve). Another use is as a food preservative. Companies add a high amount of sucrose to jams and jellies, increasing their shelf lives and decelerating several microorganism growths like bacteria and viruses.
Glucose
It is the simplest of all carbohydrates, also called dextrose. Glucose is a monosaccharide which means it constitutes a single molecule. It is the prime form of free circulating sugar in the blood of higher animals and humans, being a fundamental component of most fruits and honey. Glucose actively helps maintain cell’s proper functioning, besides handling body tissues and organs. It’s pretty hard to examine an optimal glucose level, but an increase or decrease in a blood sugar level never goes unnoticed. The aberrations in health conditions indicate the issue appearing.
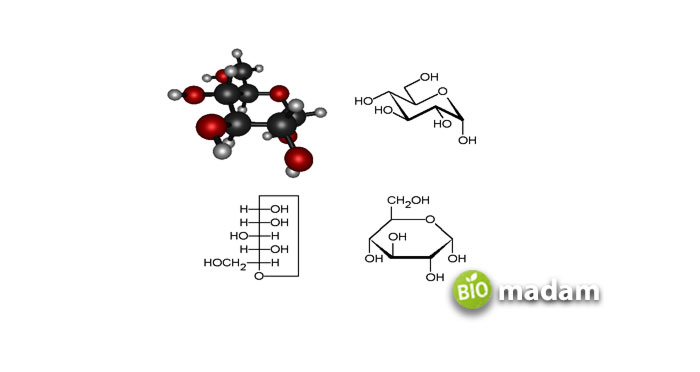
One of the most accessible examples is the lowering or increase of glucose levels in the body, termed as hypoglycemia or hyperglycemia, respectively. These conditions can observed through dizziness, consciousness, headache, and sometimes seizures. Doctors provide glucose drips to people who are sick and cannot eat or drink on their own. The most common route to administer glucose in a patient’s body is intravenous. Moreover, it is also given to patients who are severely ill due to alcohol consumption. On the other hand, if a patient is hyperglycemic, he is generally given some units of insulin to lower glucose levels.
Starch
Starch is a polysaccharide and probably the essential carbohydrate of the human body. We mainly extract it from agricultural materials, used as the principal raw resources. Since starch is biodegradable, it also serves as a suitable raw material for substituting fossil fuels. Moreover, various chemical applications use starch as their primary raw materials, such as detergents, glues, etc. They hold significant applications in the pharmaceutical and fermentation industries, and also helps in cellular respiration.
After extracting starch from agricultural products, it appears white, powdery, odorless, and tasteless. Experts extensively use it in the food industry for thickening purposes. For instance, most recipes of various soups include its usage as a thickening agent. Maltodextrin is an instead processed form of starch obtained through mild hydrolysis. This processing step helps increase the solubilization of starch. For example, starch use in sports drinks and bottle feeding formulas to provide nutrition to infants.
Acetic Acid
An organic compound having a colorless appearance and a strong, pungent odor is called acetic acid. Another name for this compound is methane carboxylic acid or ethanoic acid. We commonly use acetic acid in vinegar for daily use, which is its diluted form. Acetic acid helps produce various substrates that are further utilized in other chemical reactions in the industry. Besides, this acid works as a solvent for crystallization purposes, thereby emphasizing its utility for purifying other organic compounds. Moreover, industrialists use it as an initiating material to prepare other chemicals, including vinyl acetate, acetic anhydride, various esters, and vinegar.
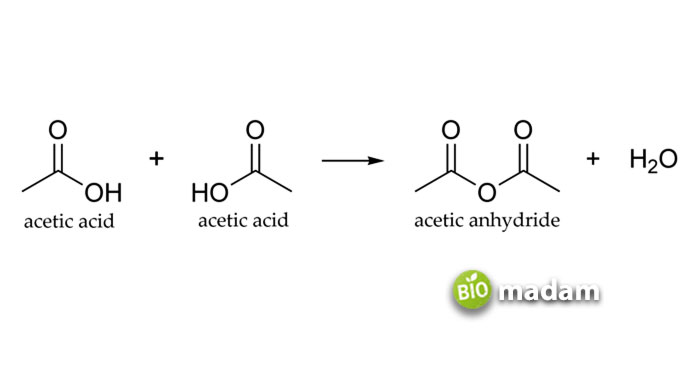
It has significant use in the field of medicine as well as it functions as an antiseptic and antimicrobial agent. Studies show its efficacy as an antimicrobial agent at a concentration of 3%. A 3-5% acetic acid solution is also used to screen putative cervical cancer patients via visual examination. Another application involves using 1-2% solution of acetic acid in water for the lysis of red blood cells to ease white blood cells visibility.
We utilize this compound in preparing pickles, condiments, and salads’ seasoning. Additionally, it also holds baking applications when mixed with alkaline substances (such as baking soda). It produces gas, providing the baked goods with a soft and puffy texture. Besides, it also assists in manufacturing inks, perfumes, various dyes, rubbers, and plastics.
Fats & Oils
These are the standard organic terms constituting essential food items of our daily consumption. Although all are macronutrients, a fat molecule still has twice the energy compared to carbohydrates and proteins when metabolized in the body. These organic substances are the primary source of stored energy. Moreover, fats and oils help a body perform several functions, such as fat-soluble vitamin circulation in a living body. Some particular fats promote various signaling processes in the body, including eicosanoids, sphingolipids, etc.
Hydrocarbons
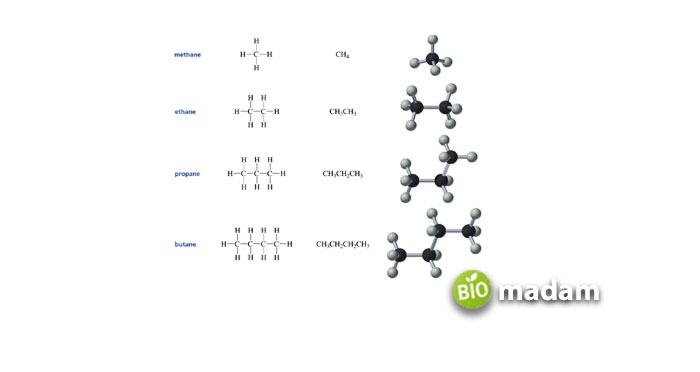
Besides consisting of only two molecules, carbon and hydrogen, hydrocarbons still contain diversity! These compounds differ in their chemical composition, the number of atoms, and distinct bonding arrangements. The simplest form of hydrocarbon is methane which is the main ingredient of natural gas. Nowadays, hydrocarbon derivatives help a building stay warm, being a great energy source. They also power up the vehicles. Other important examples of hydrocarbons for daily use are:
- Ethyne,
- Propane,
- Propyne,
- Butane,
- Butadiene,
- Ethane, etc.
Which One is the Most Abundant Organic Compound?
Now that we know the types and importance of organic compounds, it’s necessary to comprehend which one is more abundant. Carbohydrates, out of all carbon-hydrogen compounds, are the most primary and abundant form of such compounds. The reason behind this is its usage almost everywhere around us and within our bodies. Carbohydrates play several functions in the body, in glucose, sucrose, starch, etc., that we have discussed above. You can read more about the ample carbohydrate content in dragon fruit here.

Jeannie has achieved her Master’s degree in science and technology and is further pursuing a Ph.D. She desires to provide you the validated knowledge about science, technology, and the environment through writing articles.

